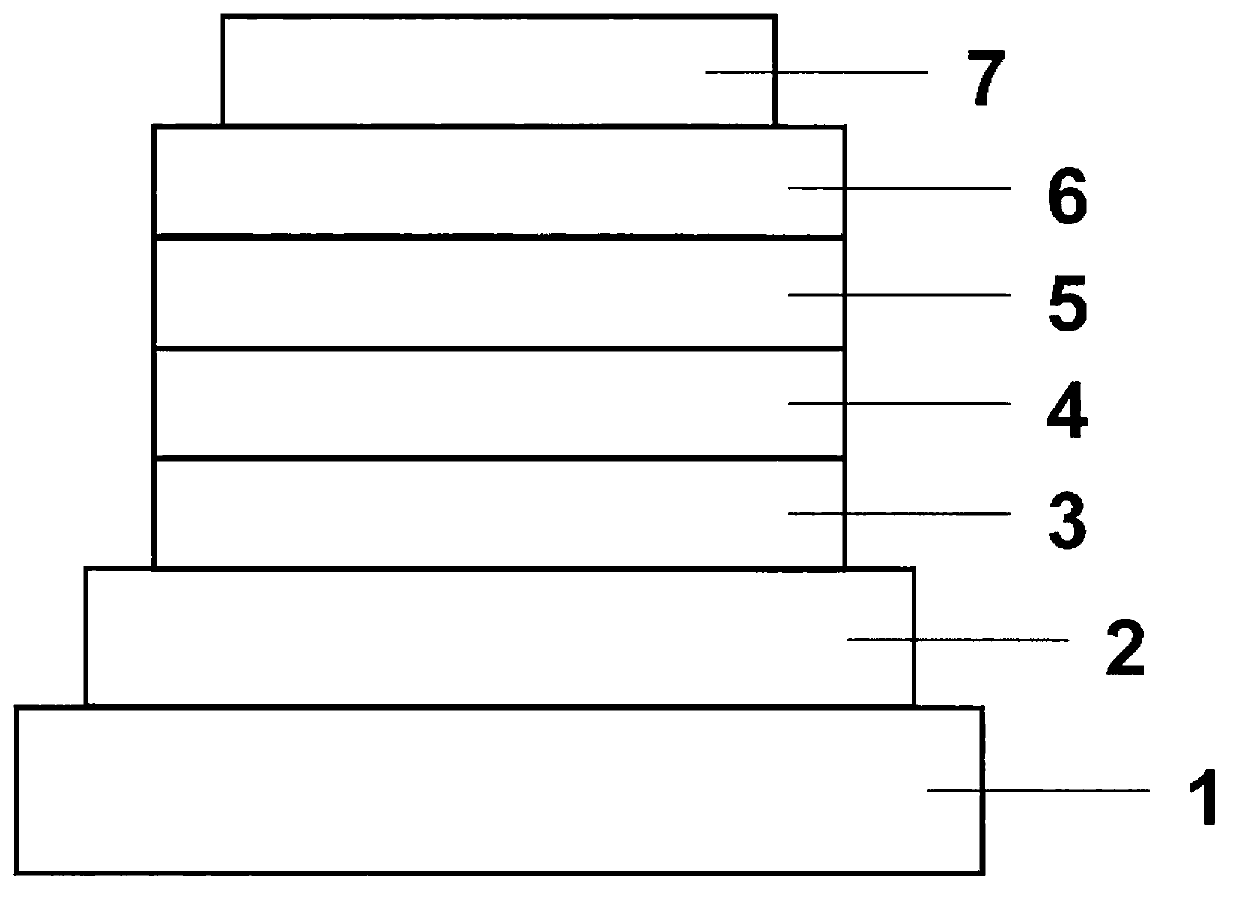
Organic electroluminescent element
An electroluminescence element and an electroluminescence technology, which are applied in the directions of electroluminescence light sources, electrical components, electric light sources, etc., and can solve the problems of undisclosed compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0118] Hereinafter, the present invention will be described in more detail using examples. Of course, the present invention is not limited to these examples, and can be implemented in various forms as long as the gist thereof is not exceeded.
[0119] The indolocarbazole compound used in the present invention was synthesized according to the scheme shown below. In addition, the compound number corresponds to the number attached to the above-mentioned chemical formula.
Synthetic example 1
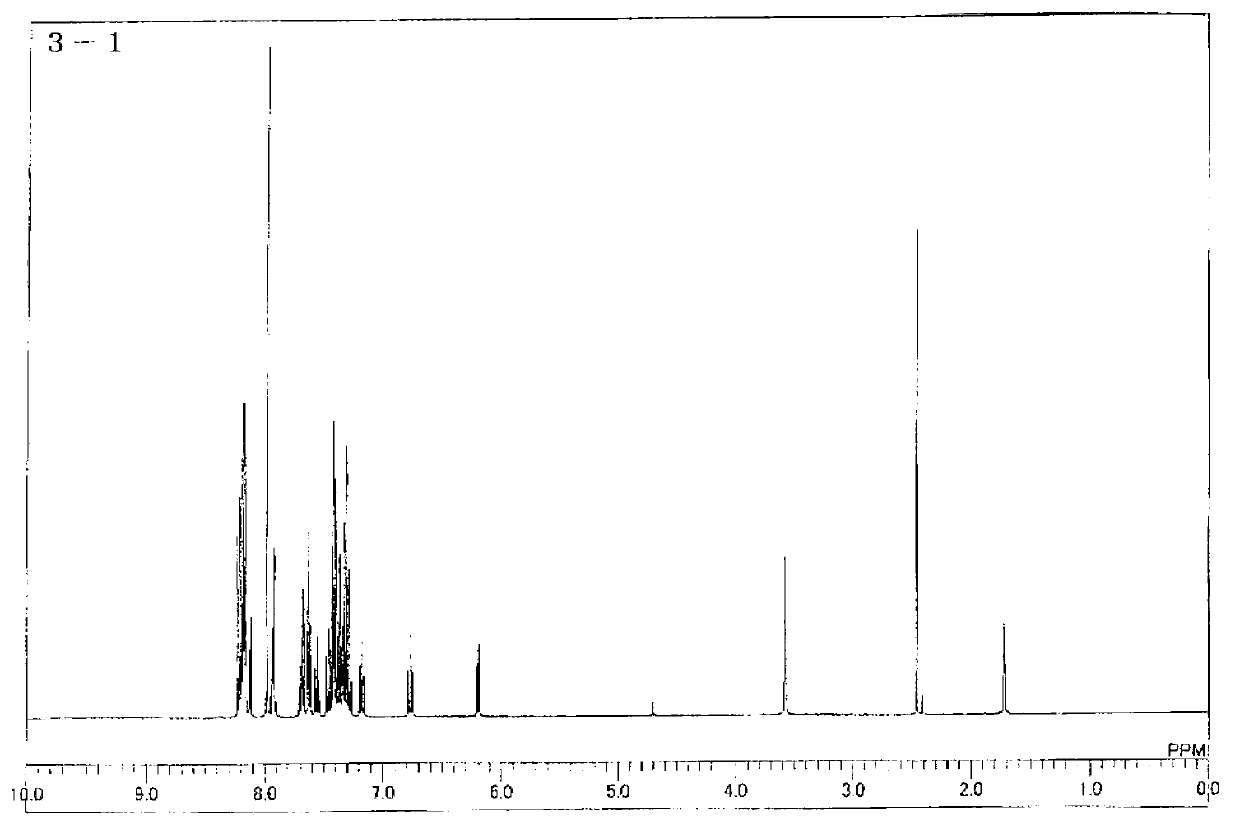
[0121] Synthesis of Compound 3-1
[0122]
[0123] Under a nitrogen atmosphere, 10.0 g (0.039 moles) of 5,12-dihydroindolo[3,2-a]carbazole (IC-1), 39.8 g (0.20 moles) of iodobenzene, and 6.2 g (0.098 moles) of copper were added. mol), 8.1 g (0.059 mol) of potassium carbonate, and 200 ml of tetraethylene glycol dimethyl ether were stirred. Then, it heated to 190 degreeC, and stirred for 24 hours. After cooling the reaction solution to room temperature, copper and inorganic substances were collected by filtration. 200 ml of water was added to the filtrate, stirred, and the precipitated crystals were collected by filtration. After drying this under reduced pressure, it was purified by column chromatography to obtain 9.7 g (0.029 mol, yield: 75%) of Intermediate A as a white powder.
[0124] Under a nitrogen atmosphere, 25.0 g (0.075 moles) of intermediate A, 25.6 g (0.066 moles) of 4-(3-bromophenyl)-2,6-diphenylpyridine, and 25.5 g (0.13 moles) of copper iodide were added ...
Synthetic example 2
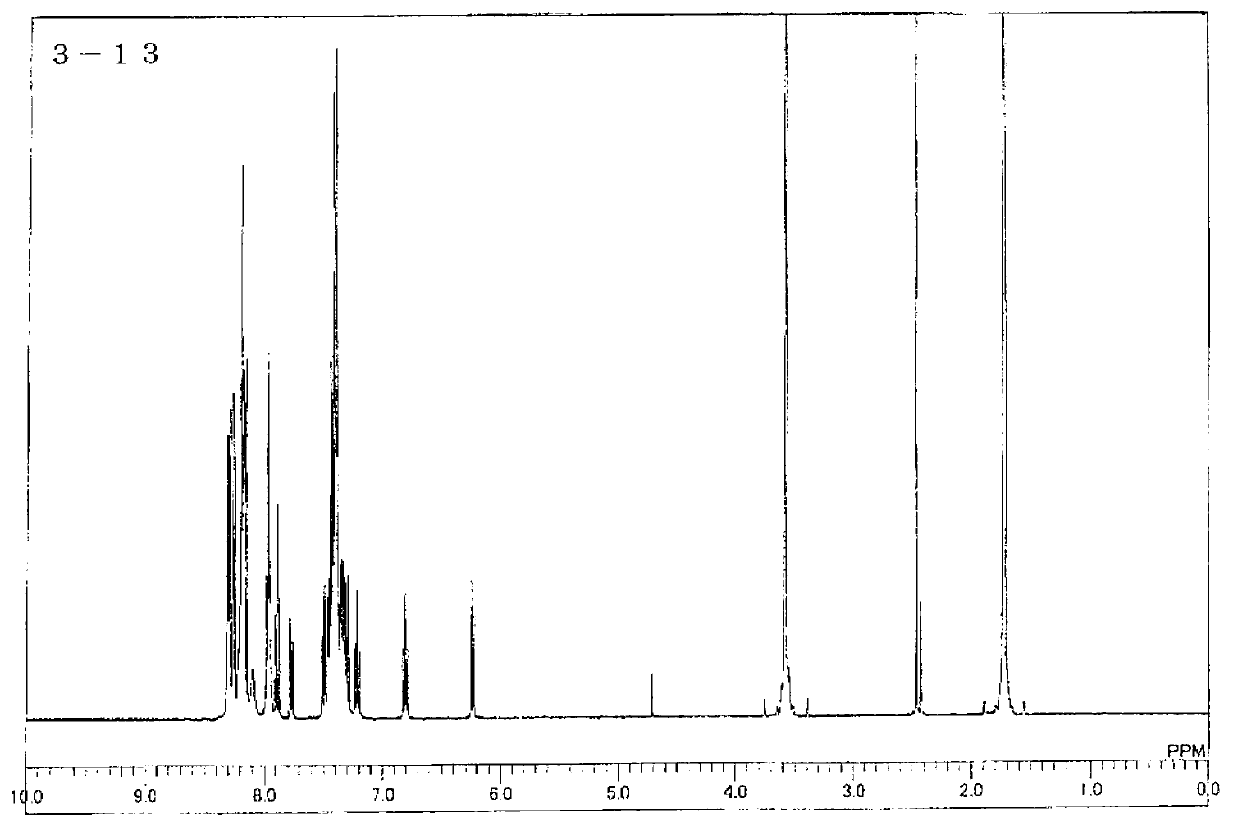
[0127] Synthesis of Compound 3-13
[0128]
[0129] Under a nitrogen atmosphere, add IC-19.9g (0.039 mol), 4-(3-bromophenyl)-2,6-diphenylpyridine 14.6g (0.038 mol), copper iodide 13.5g (0.071 mol), 16.6 g (0.12 mol) of potassium carbonate and 350 ml of 1,3-dimethyl-2-imidazolidinone were stirred at 185° C. for 30 hours. After cooling the reaction solution to room temperature, inorganic substances were collected by filtration. The obtained filtrate was added to 4000 ml of water, stirred, and the precipitated crystal was collected by filtration. After drying under reduced pressure, it was purified by column chromatography to obtain 20.5 g (0.036 mol, yield: 94%) of Intermediate B as a white powder.
[0130] Under a nitrogen atmosphere, 19.1 g (0.034 mol) of intermediate B, 12.9 g (0.034 mol) of 4-(3-bromophenyl)-2,6-diphenylpyridine, and 12.2 g (0.064 mol) of copper iodide were added , 15.8 g (0.12 mol) of potassium carbonate, and 300 ml of 1,3-dimethyl-2-imidazolidinone w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| visible light transmittance | aaaaa | aaaaa |
| coating thickness | aaaaa | aaaaa |
| coating thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com