Monoclonal antibody recongnizing epitope for HCV (hepatitis C virus) NS3 helicase ATP (adenosine triphosphate) binding site
A hepatitis C virus, monoclonal antibody technology, applied in the direction of antibodies, antiviral agents, antibody medical components, etc., can solve the problems of complicated operation, inability to be widely used, expensive, etc., and achieve the effect of good specific recognition ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032] The present invention will be further described below in combination with specific experiments, but it is not limited thereto.
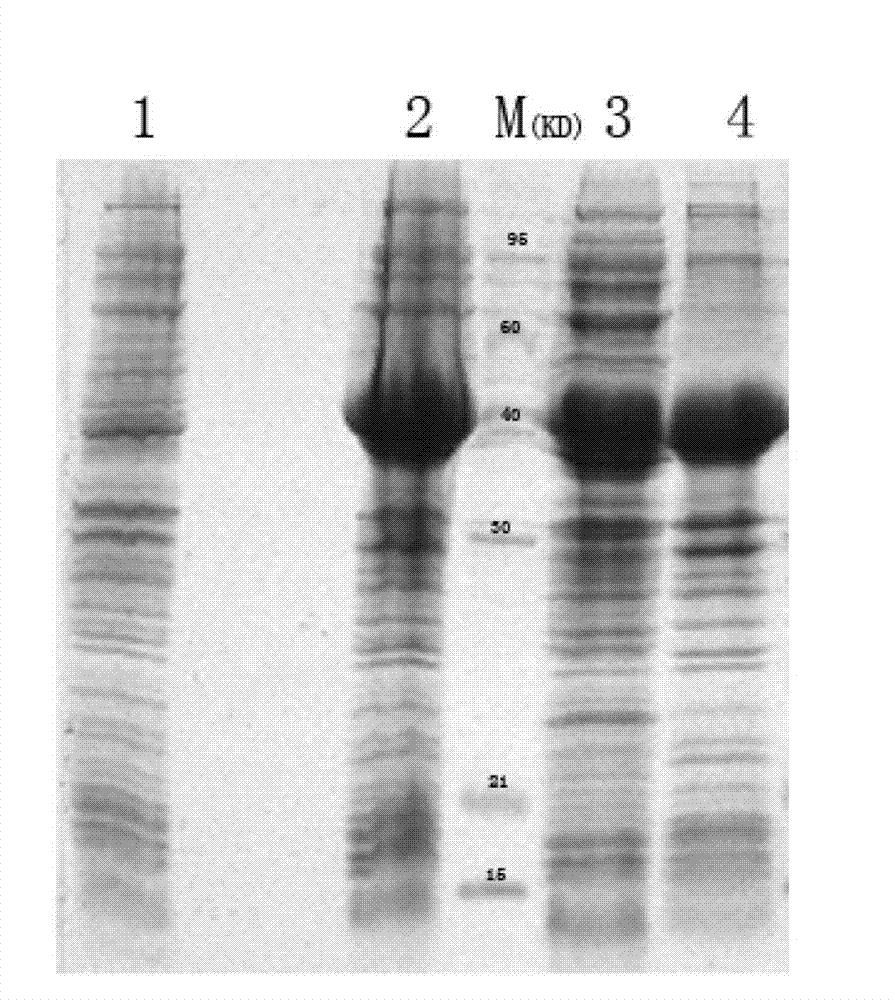
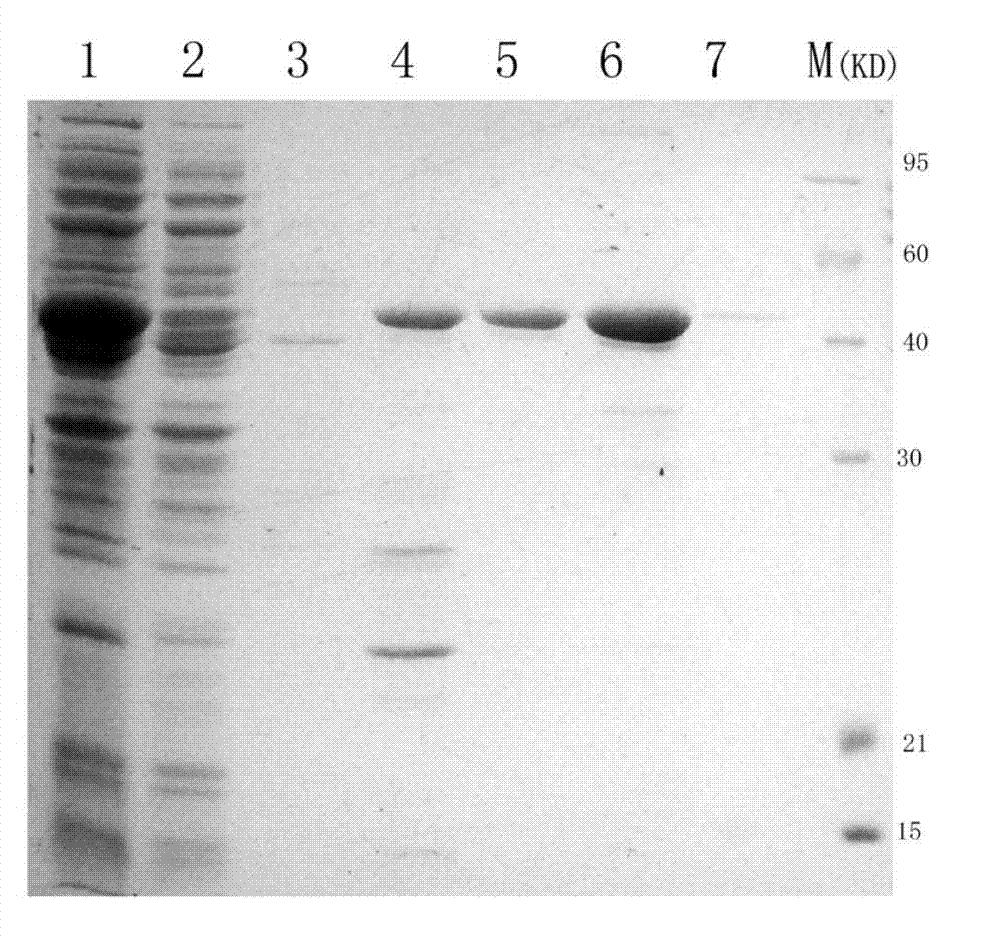
[0033] Referring to the literature, the truncated HCV NS3 (1192aa~1459aa) belongs to the immunologically active region, and was determined as the target protein to be expressed. The HCV NS3 protein can be prepared by genetic engineering, or the purified HCV NS3 protein can be purchased directly.
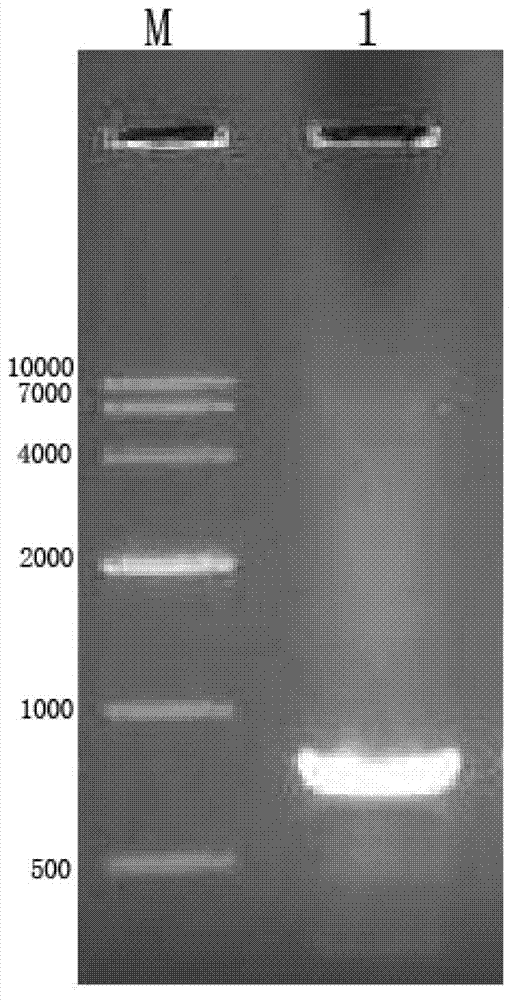
[0034] The gene method is as follows: the DNA sequence encoding HCV NS3 (1192aa~1459aa) is cloned by PCR method, and then inserted into the multi-cloning site of the expression vector to construct the recombinant expression plasmid, and then transformed into the expression host bacteria to construct the engineering Bacterial strains, engineered strains are induced to express, isolated and purified to obtain HCV NS3 recombinant protein. This specific method is many kinds, and a specific embodiment is given as an example below:
[0035] Constructio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com