Method and apparatus for preparing novel liposome
A technology of liposomes and proliposomes, which is applied in the field of preparation of liposome preparations and devices for preparing liposomes, and can solve problems such as inability to apply
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a~1f
[0053] Example 1a~1f and 2a~2d: Preparation of amphotericin B liposomes
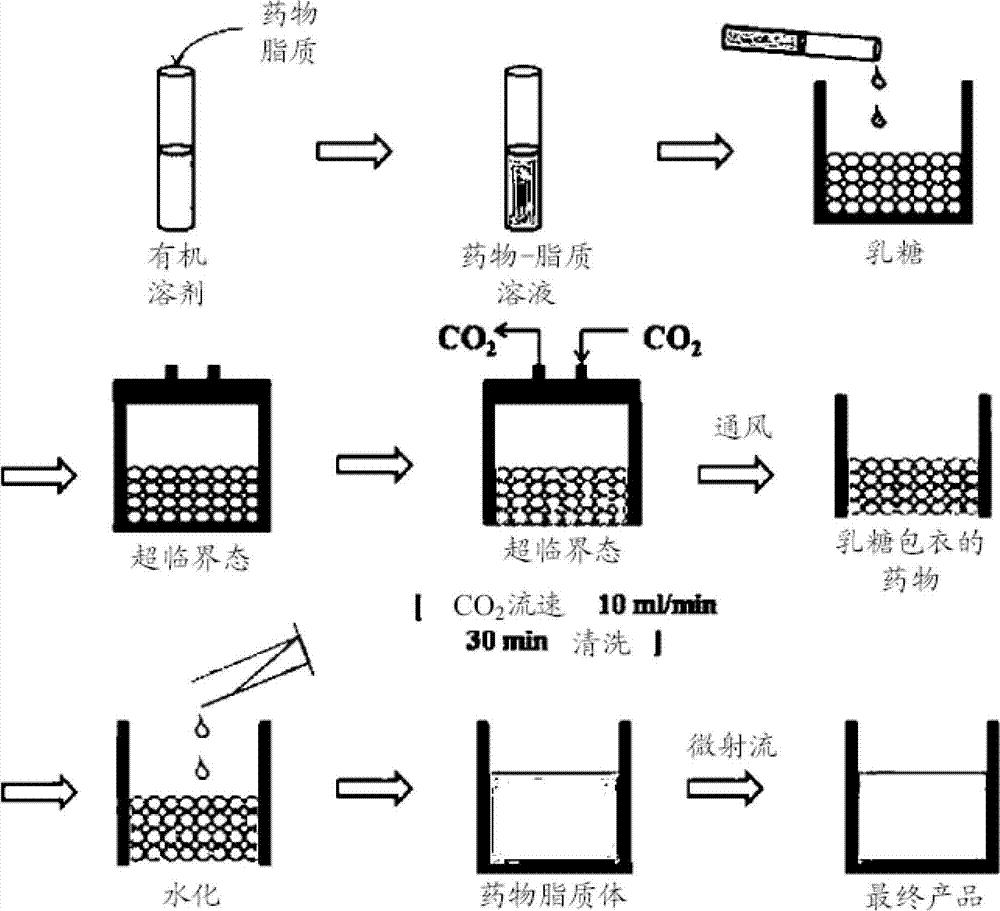
[0054] Dissolve 84 mg of distearoylphosphatidylcholine (DSPC) in 1 ml of a 1:1 mixed solvent of chloroform and methanol at 65°C. 200 mg of ascorbic acid (VIt-C) was completely dissolved in 2 ml of N,N-dimethylacetamide (DMA) by ultrasound for 10 minutes. 50 mg of amphotericin B was dissolved in the DMA-Vit C solution at 65°C, and the DSPC solution was added to the resulting solution. At 65°C, 213 mg of hydrogenated soybean phosphatidylcholine and 52 mg of cholesterol were dissolved in 1 ml of a 1:1 mixed solvent of chloroform and methanol. The solution of hydrogenated soybean phosphatidylcholine and cholesterol was mixed with the amphotericin B-DSPG solution.
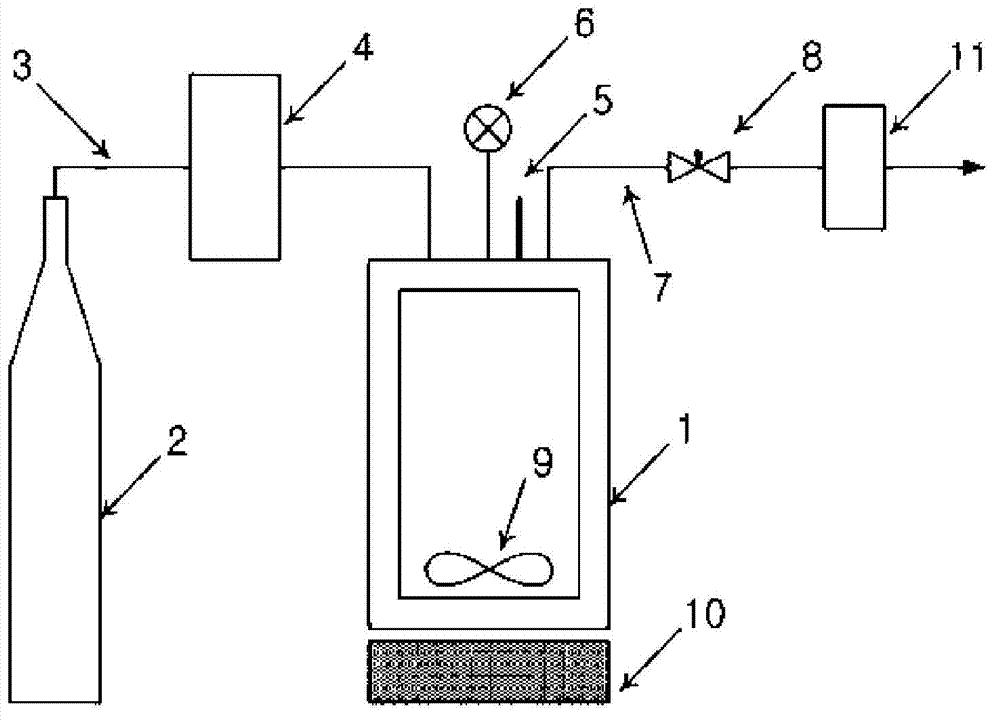
[0055] The amphotericin B-lipid solution and 900 mg of lactose were contained in the reaction vessel 1, and then sealed. The temperature of the reaction vessel was maintained at 45°C, 55°C and 65°C. By operating the pump 4, supercritical carbon dio...
Embodiment 1
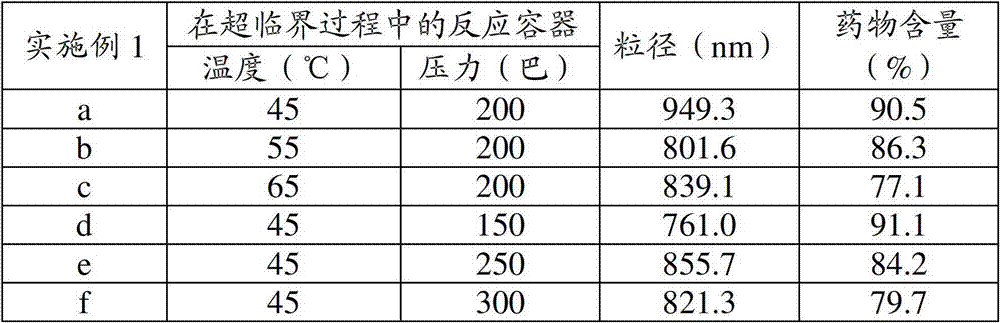
[0057] Example 1 relates to the characteristics of liposome solution according to the temperature and pressure of the reaction vessel during the supercritical process. In Examples 1a to 1f, the particle size and the drug content according to the temperature and pressure of the reaction vessel are recorded in Table 1. Example 2 relates to: when the proliposome particles prepared at a reaction temperature of 45°C and a pressure of 200 bar are hydrated by adding an aqueous solution (including water) in the supercritical process corresponding to Example 1a, the lipid Body solution is based on the characteristics of hydration temperature. In Examples 2a to 2d, the particle size, drug content, and formation / non-formation of liposomes according to the hydration temperature are recorded in Table 2.
[0058] Table 1
[0059] [Table 1]
[0060] [table]
[0061] In Examples 1a to 1f, the particle size and drug content according to the temperature and pressure of the reaction vessel
[0062]
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com