Pyrophosphate compound and method for producing same
A technology for pyrophosphate compounds and manufacturing methods, which is applied in the fields of phosphorus compounds, chemical instruments and methods, and final product manufacturing. It can solve the problems of mixed-in synthesis, difficulty in removing impurities, etc., and achieve the effect of easy synthesis and inhibition of mixing-in
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~4 and comparative example 1~3
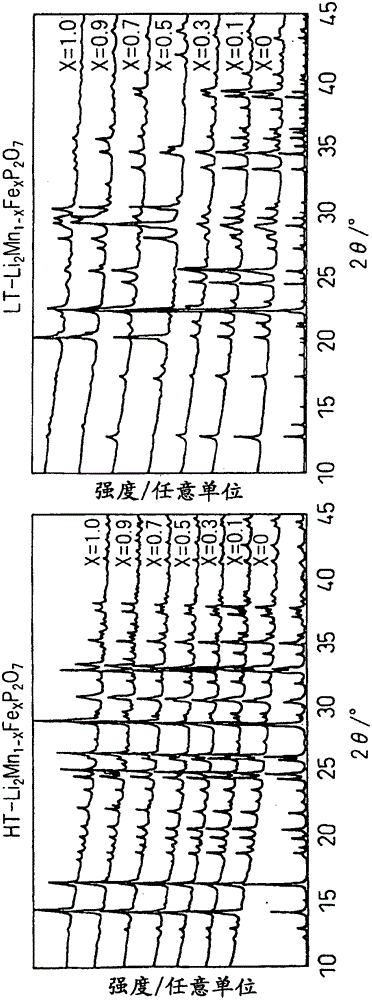
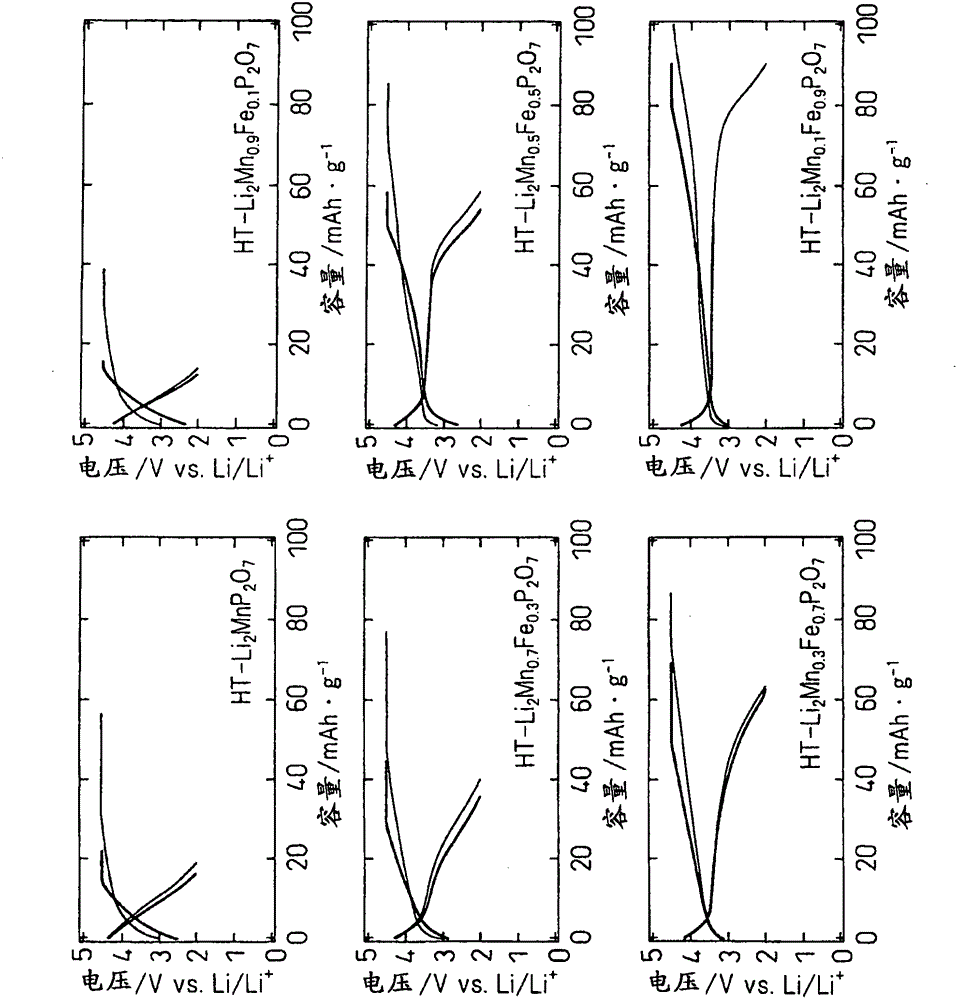
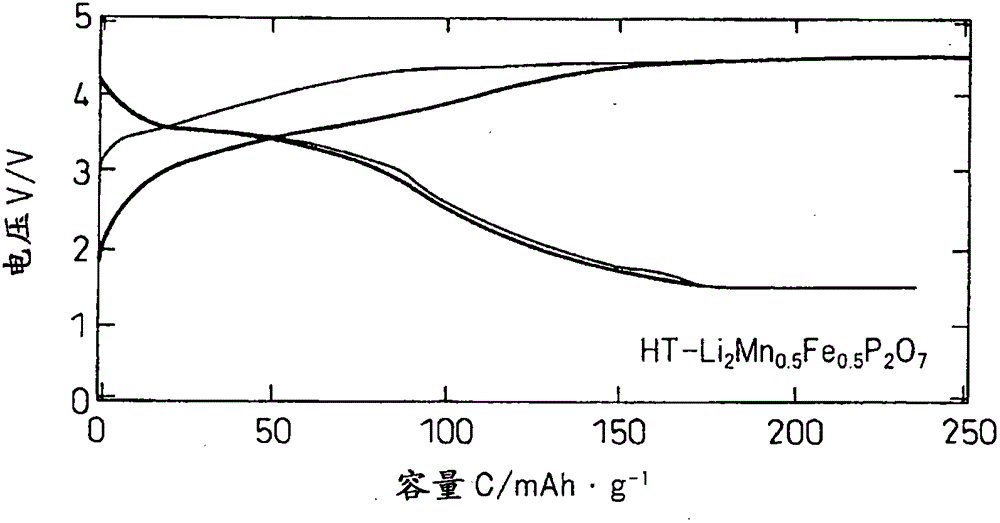
[0049] to make Li 2 mn 1-x Fe x P 2 o 7 (0≤x≤1) becomes the mode of the stoichiometric ratio shown in Table 1, weighing lithium carbonate (Li 2 CO 3 ), manganese oxalate hydrate (MnC 2 o 4 0.5H 2 O), iron oxalate hydrate (FeC 2 o 4 2H 2 O), ammonium hydrogen phosphate [(NH 4 ) 2 HPO 4 ], using acetone as a solvent, wet pulverization and mixing with a ball mill at 240 rpm for 30 minutes and at 480 rpm for 2 hours. The mixed slurry was dried under reduced pressure at room temperature to obtain a precursor. It was fired at 600°C for 6 hours in an Ar atmosphere to obtain a high-temperature phase of HT-Li 2 mn 1-x Fe x P 2 o 7 (0≤x≤1), fired at 450°C for 24 hours, thus obtaining the low-temperature phase of LT-Li 2 mn 1-x Fe x P 2 o 7 (0≤x≤1).
[0050] [Table 1]
[0051]
[0052] (structural analysis)
[0053] Powder X-ray diffraction measurements used CuKα as the line source. Structural analysis of the product, identification of impurities, and prese...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com