Preparation methods of substituted diaryl phenol, organophosphorus ester and organic phosphate
A technology of organic phosphate and aryl phenol, which is applied in the field of organic phosphate preparation, can solve problems such as pollution and unfavorable environmental protection, and achieve the effects of overcoming pollution, benefiting environmental protection, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
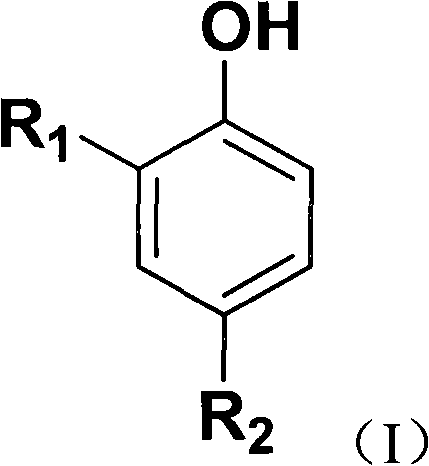
[0013] According to the preparation method of substituted diarylphenol of the present invention, in the step (1), the amount of methylal added is, in molar ratio, substituted arylphenol:methylal=1:1-10; Preferably, substituted arylphenol:methylal=1:7-10; more preferably, substituted arylphenol:methylal=1:8-9.
[0014] According to the preparation method of substituted diarylphenols of the present invention, the bridging reaction conditions include: the temperature is 5°C-145°C, and the time is 1-36 hours; preferably, the temperature is 35-80°C, and the time is 3-12 hours. hours; more preferably, the temperature is 60-62° C., and the time is 5-6 hours.
[0015] The catalyst is one or more selected from sulfuric acid, p-toluenesulfonic acid, benzenesulfonic acid, phosphoric acid, and nitric acid; preferably, sulfuric acid, p-toluenesulfonic acid, and benzenesulfonic acid with a concentration of more than 95% by weight one or more of acids; more preferably, one or more of sulfur...
Embodiment 1
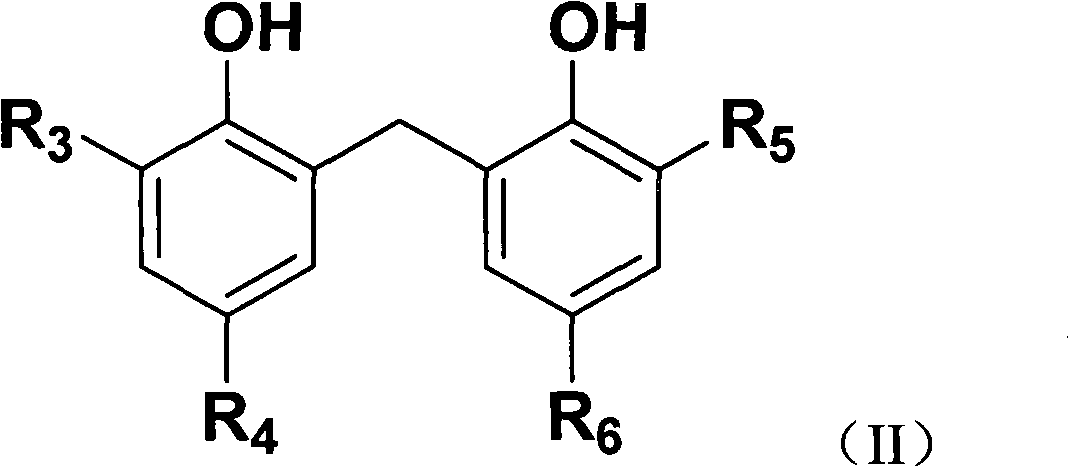
[0041] Preparation of substituted diarylphenols Add 41.27 grams of 2,4-di-tert-butylphenol and 150 ml of methylal into the reaction flask, stir until the solid is fully dissolved, slowly add 5 ml of 98% sulfuric acid at room temperature, and dropwise complete Then react at 62°C for 6 hours; then cool down to room temperature, add anhydrous calcium chloride to dehumidify the moisture in the reaction system, then filter, distill to obtain a solid product, and then wash with a large amount of water to adjust the pH to neutral, the obtained Solid product, liquid phase removed. After drying at 80°C, 42.4 g of white solid 2,2'-methylene-bis(4,6-di-tert-butylphenol) was obtained, and the yield based on the amount of 2,4-di-tert-butylphenol was 99.8%, melting point>158°C.
[0042] Preparation of organophosphate: add 42.4 g of the obtained white solid 2,2'-methylene-bis(4,6-di-tert-butylphenol) into the reaction flask, then add 160 ml of toluene and 60 ml of triethylamine, stir When ...
Embodiment 2
[0047] Preparation of substituted diarylphenol: Add 41.35 grams of 4-methyl-6-tert-butylphenol and 160ml of methylal into the reaction kettle, stir until the solid is fully dissolved, and slowly add p-toluenesulfonate at room temperature 3 grams of acid, after the dropwise addition, react at 60°C for 5 hours; then cool down to room temperature, add anhydrous calcium chloride to dehumidify the moisture in the reaction system, wash with a large amount of water to adjust the pH to neutral, and then filter , Distill to obtain a solid product, and then wash the obtained solid product with water to remove the liquid phase. After drying at 80°C, 42.6 g of white solid 2,2'-methylene-bis(4-methyl-6-tert-butylphenol) was obtained, based on the amount of 4-methyl-6-tert-butylphenol The yield was 99.5%, and the melting point was >140°C.
[0048] Preparation of organic phosphate: the white solid 2,2'-methylene-bis(4,6-di-tert-butylphenol) 42.6 is added in the reaction flask, then 160ml of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com