Organic semiconductor materials, their preparation methods and applications
A technology of organic semiconductors and organic palladium, which is applied in semiconductor/solid-state device manufacturing, semiconductor devices, organic chemistry, etc., can solve the problems of blue light-emitting materials such as poor luminous efficiency and stable carrier transport performance, and achieve excellent solubility , Improve the stability of performance, improve the effect of electron transfer rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The embodiment of the present invention further provides the method for preparing the above-mentioned organic semiconductor material, including the following steps:
[0029] Step S01, respectively providing boric acid of structural formula I or boric acid ester of structural formula II, and tripolyindene monomer of structural formula III;
[0030] Structural formula I is: Structural formula II is: Structural formula III is:
[0031] Step S02, in an inert atmosphere, dissolve the triindene monomer of the structural formula III and the boric acid of the structural formula I or the boric acid ester of the structural formula II in an organic solvent at a molar ratio of 1:3-4.5, and perform a Suzuki reaction to obtain an organic Semiconductor material precursor, structural formula (IV) is as follows:
[0032]
[0033] Step S03, in an inert atmosphere, at a temperature of -78 to 180°C, dissolve the organic semiconductor material precursor in an organic solvent, and ...
Embodiment 1
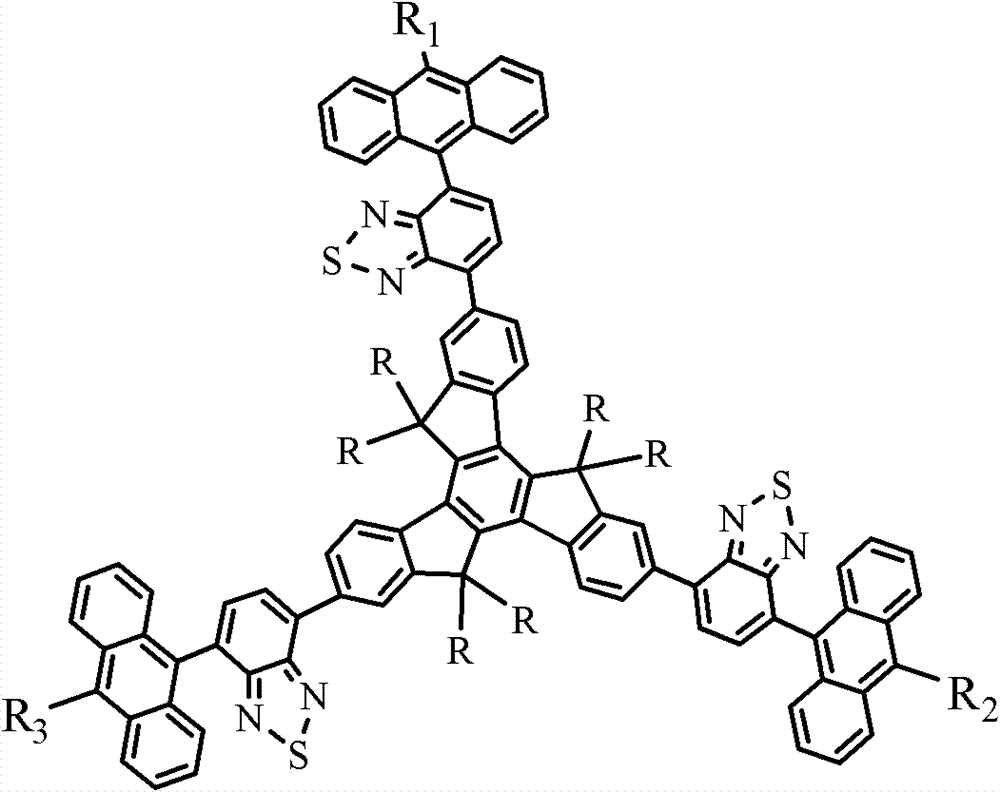
[0074] Example of the present invention 2-(5-(10-cyano-anthracene-9-yl)benzothiadiazol-2-yl)-7,12-bis(anthracene-9-yl)benzothiadiazole- 2-yl)-5,5',10,10',15,15'-hexaethyltriindene (CNAB-ET) has the following structural formula:
[0075]
[0076] Example of the present invention 2-(5-(10-cyano-anthracene-9-yl)benzothiadiazol-2-yl)-7,12-bis(anthracene-9-yl)benzothiadiazole- 2-yl)-5,5',10,10',15,15'-hexaethyltripolyindene (CNAB-ET) preparation method, comprises the steps:
[0077] Step 1, prepare tripolyindene:
[0078] i. Add 10 mmol of 1-indanone to a mixed solution containing 8 mL of acetic acid and 4 mL of concentrated hydrochloric acid, heat to 100° C., and stir under reflux for 20 hours. After the reaction, the reaction solution was poured into a beaker filled with ice water, resulting in a large amount of solid precipitation. After the precipitate was washed with water, acetone and dichloromethane in sequence, a white solid powder, that is, trisindene, was obtained, ...
Embodiment 2
[0103] Examples of the present invention 2,7,12-tris(5-(10-cyano-anthracene-9-yl)benzothiadiazol-2-yl)-5,5',10,10',15,15 '-hexaethyltripolyindene (CN3AB-ET) has the following structural formula:
[0104]
[0105] Examples of the present invention 2,7,12-tris(5-(10-cyano-anthracene-9-yl)benzothiadiazol-2-yl)-5,5',10,10',15,15 '-hexaethyl tripolyindene (CN3AB-ET) preparation method, comprises the steps:
[0106] Step 1 is with reference to step 1 in embodiment 1; Wherein, step Ⅲ is:
[0107] Preparation of 2,7,12-tribromo-5,5′,10,10′,15,15′-hexaethyltripolyindene:
[0108] Dissolve 5mmol of 5,5′,10,10′,15,15′-hexaethyltripolyindene in 25mL of dichloromethane, and at the same time dissolve 1mL of liquid bromine in 10mL of dichloromethane, at 0°C Add the dichloromethane solution containing liquid bromine to the dichloromethane solution containing 5,5′,10,10′,15,15′-hexaethyltripolyindene, adjust the system temperature to room temperature, avoid Light reaction for 12 hours. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com