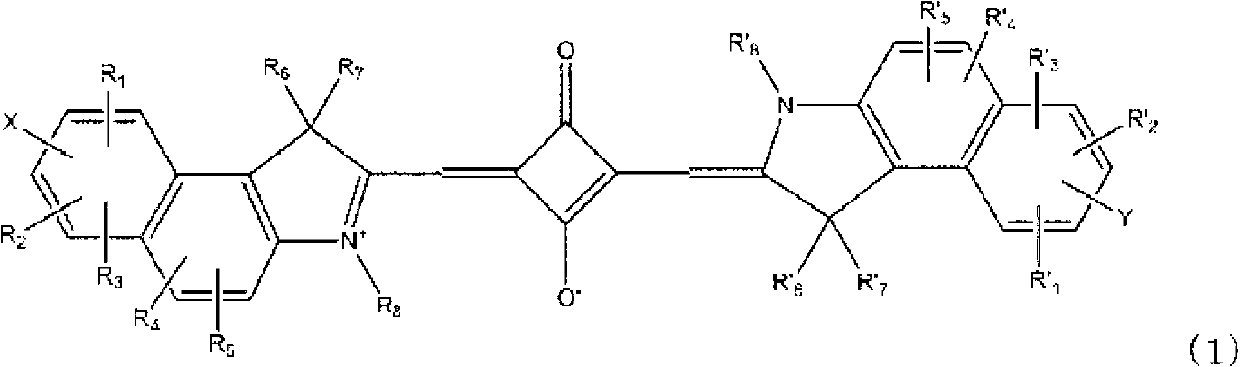
Squarylium dye, dye-sensitized solar cell using the dye, and photoelectric conversion element using the dye
A technology of squarylium and pigment, applied in the field of pigment-sensitized solar cells, capable of solving problems such as undisclosed benzindole compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
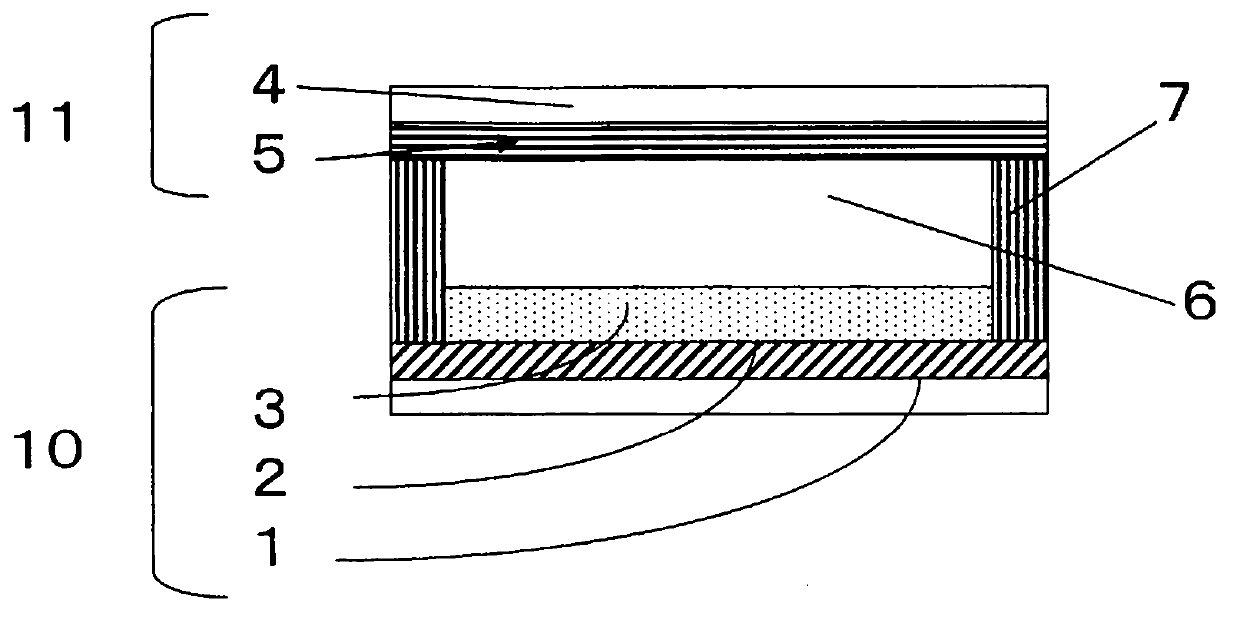
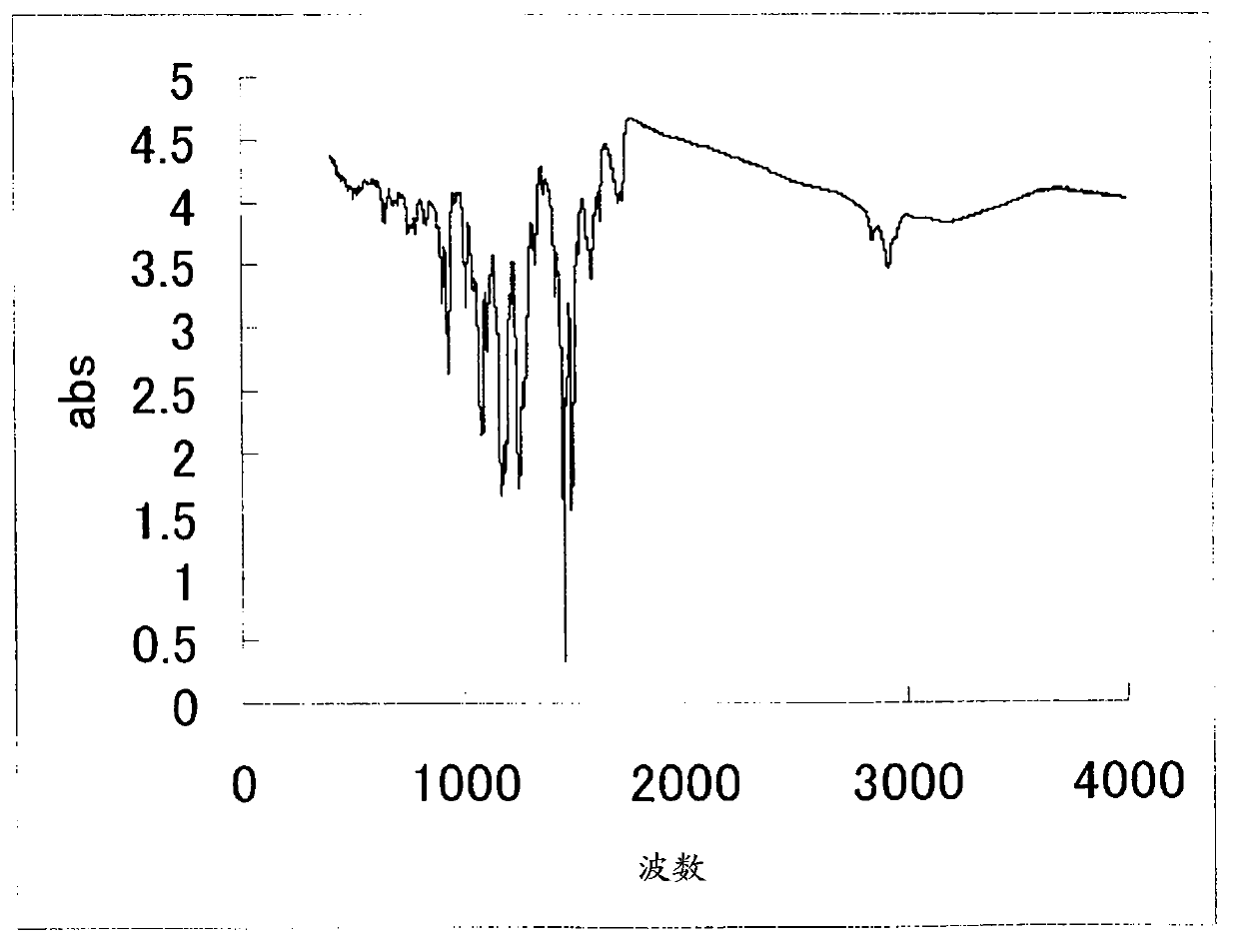
Image
Examples
Embodiment
[0104] Hereinafter, the present invention will be described in more detail based on Examples and Comparative Examples. It should be noted that Synthesis Examples 1 to 9 and 13 can be understood as examples (excluding the dye D-6).
Synthetic example 1
[0106]
[0107] Ethoxycarbonylbenzoindolenine, using 1-bromo-4-ethoxycarbonylnaphthalene, benzophenone hydrazone, palladium acetate, BINAP, sodium butoxide, methyl isopropyl ketone, p-toluenesulfonic acid Commercially available reagents were used for synthesis. 78.87 mmol of 1-bromo-4-ethoxycarbonylnaphthalene was dissolved in a toluene solvent, palladium acetate and BINAP were added, and then 78.87 mol of benzohydrazone and sodium butoxide were added to react overnight at 80°C. Further, 123.46 mmol of methyl isopropyl ketone and p-toluenesulfonic acid monohydrate were added and reacted overnight in an ethanol solvent to obtain the target benzoindolenine ester in a yield of 30%.
Synthetic example 2
[0109] For the synthesis of N-n-alkylethoxycarbonylbenzoindolenine salt, dissolve 2,3,3-trimethyl-6-ethoxycarbonylbenzoindolenine 0.1mol and 1-Iodoethane was reacted for 7 hours under reflux. The solid content was filtered to obtain 1-n-ethyl-2,3,3-trimethyl-6-ethoxycarbonylbenzoindolenine iodide in a yield of 55%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com