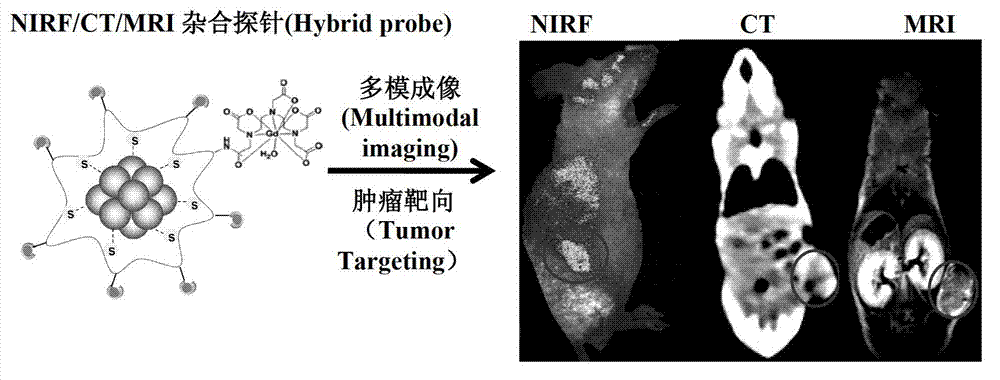
Contrast agent and preparation method thereof
A technology of contrast agent and mixture, which is applied in the field of medical imaging and can solve the problems of reduced efficiency and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
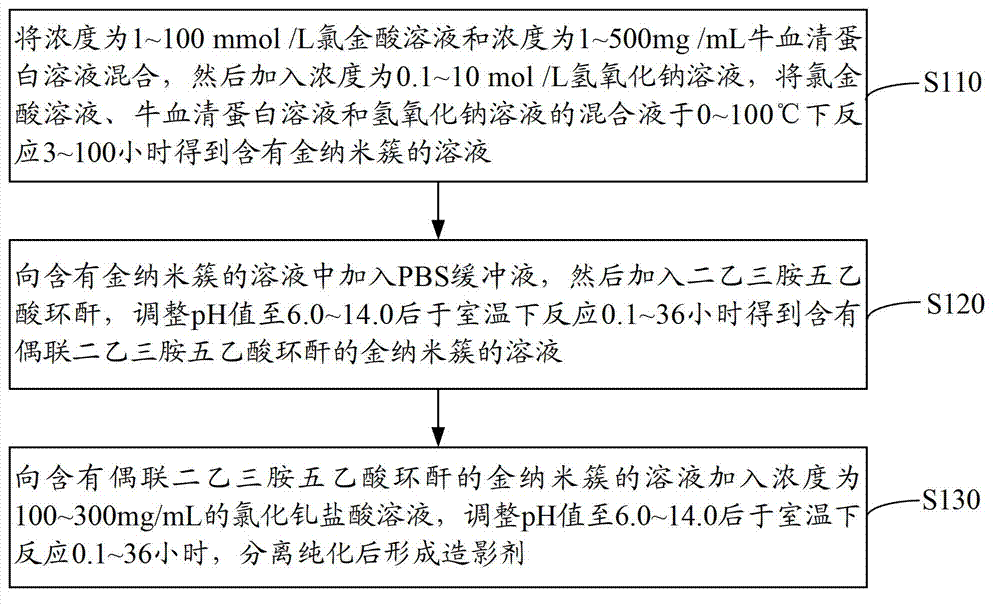
[0037] see figure 1 , a method for preparing a contrast agent, comprising the steps of:
[0038] Step S110: mix the chloroauric acid solution with a concentration of 1-100mmol / L and the bovine serum albumin solution with a concentration of 1-500mg / mL, then add a sodium hydroxide solution with a concentration of 0.1-10mol / L, and dissolve the chloroauric acid solution , the mixed solution of bovine serum albumin solution and sodium hydroxide solution was shaken and reacted for 3 to 100 hours at 0 to 100°C to obtain a solution containing gold nanoclusters, wherein the volume of chloroauric acid, bovine serum albumin and sodium hydroxide solution The ratio is 0.1~5:0.1~25:0.1~2.
[0039] Mixed chloroauric acid (HAuCl 4 ) solution and bovine serum albumin (BSA) solution, and then add sodium hydroxide solution to make chloroauric acid (HAuCl 4 ) solution, bovine serum albumin (BSA) solution and sodium hydroxide solution mixture is alkaline.
[0040] Using bovine serum albumin (B...
Embodiment 1
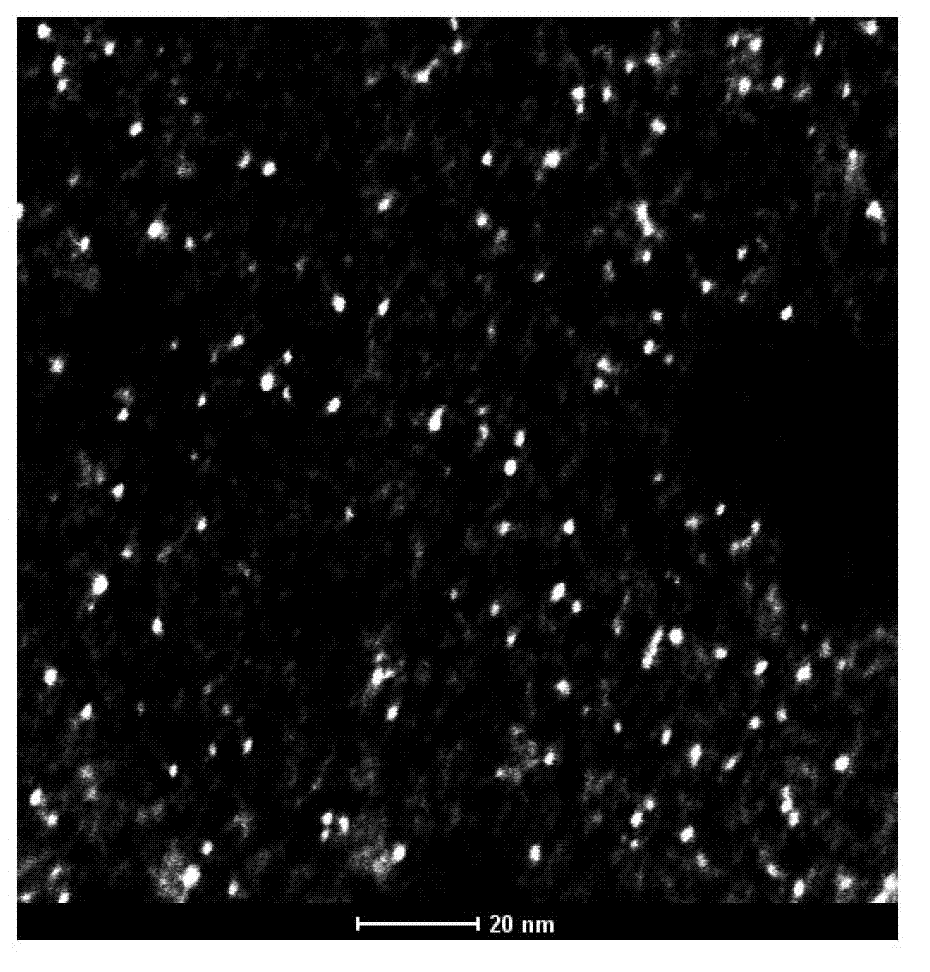
[0056] 1. Preparation of gold nanoclusters: Add 5mL of chloroauric acid solution (10mmol / L) to 5mL of BSA solution (50mg / mL, mix well, then add 0.5mL of 1mol / L NaOH solution, and place the mixture at 37°C Gold nanoclusters formed after incubation in a shaker (200 rpm) for 12 hours.
[0057] 2. Preparation of gold nanoclusters coupled with diethylenetriaminepentaacetic acid: Add 2mL of 2M PBS buffer (pH=9) to 40ml of a solution containing gold nanoclusters, then add 280mg of DTPACA powder, and use 5M NaOH solution Titrate, adjust the pH value to 6.0, and shake gently at room temperature for 2 hours to generate gold nanoclusters coupled with diethylenetriaminepentaacetic acid.
[0058] 3. Preparation of contrast agent: weigh 300mg gadolinium chloride (GdCl 3 .6H 2 O) Dissolve in 1mL of 1M HCl, and add to the above solution containing gold nanoclusters coupled with diethylenetriaminepentaacetic acid, titrate with 5M NaOH solution, adjust the pH value to 6.0, shake gently at roo...
Embodiment 2
[0067] Preparation of contrast agent
[0068] 1. Preparation of gold nanoclusters: Add 10 mL of chloroauric acid solution (5 mmol / L) into 10 mL of BSA solution (25 mg / mL), mix well, then add 1 mL of 0.5 mol / L NaOH solution, and place the mixture at 50 Gold nanoclusters were formed after incubation in a shaker at 300 rpm for 24 hours.
[0069] 2. Preparation of gold nanoclusters coupled with diethylenetriaminepentaacetic acid: add 2mL of 4M PBS (pH=9) to 50ml of gold nanocluster solution, then add 400mg of DTPACA powder, and titrate with 3M NaOH solution to adjust the pH value to 9.0, the reaction was gently shaken at room temperature for 1 hour to generate gold nanoclusters coupled with diethylenetriaminepentaacetic acid.
[0070] 3. Preparation of contrast agent: weigh 500mg gadolinium chloride (GdCl 3 .6H 2O) Dissolve in 0.5 mL of 1M HCl, and add to the above solution containing gold nanoclusters coupled with diethylenetriaminepentaacetic acid, titrate with 3M NaOH soluti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com