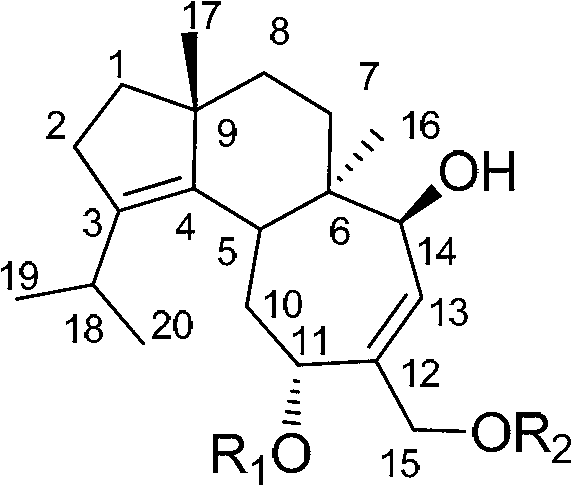
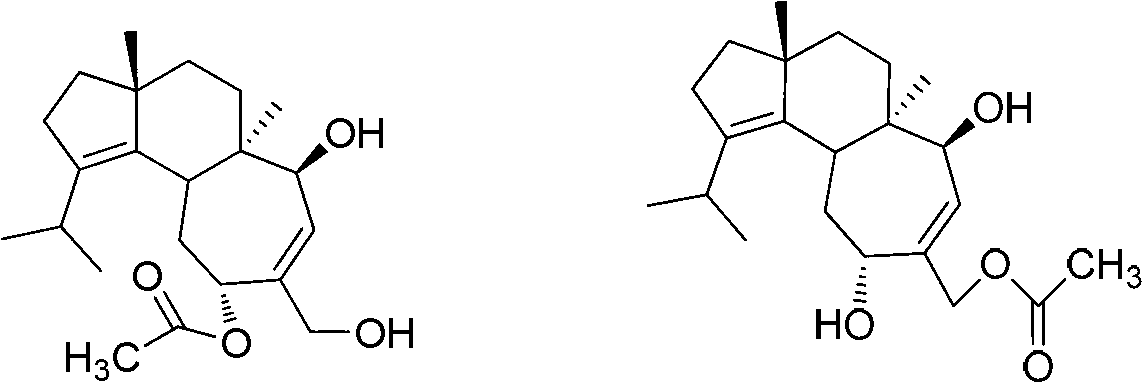
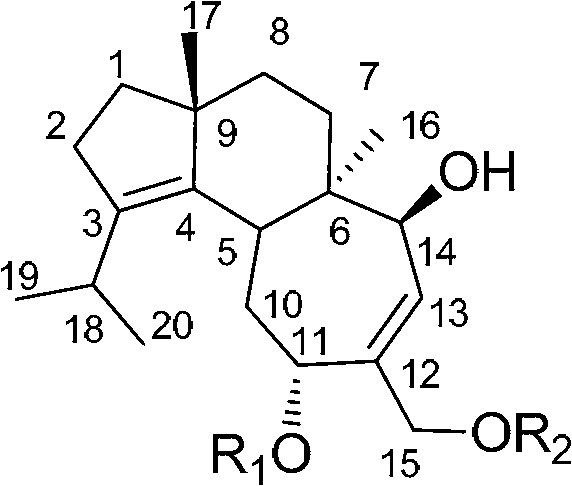
Application of cyathane diterpene compound in antitumor drug
A compound and drug technology, applied in the application field of ornithane diterpenoids in the preparation of anti-tumor drugs, can solve the problems that have not been reported on the anti-tumor activity of this type of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1. The acquisition of the strain Cyathus africanus L38 producing cyathane diterpenoids 11-O-acetylcyathatriol and 15-O-acetylcyathatriol
[0026] 1. Isolation of African black egg nest (Cyathus africanus) L38
[0027] On the basis of the high-throughput screening of active compounds of microbial secondary metabolites, the inventor obtained a solid fermented extract of Cyathus africanus isolated from the fruiting body of Cyathus africanus in Wutai Mountain, Shanxi Province. Antitumor activity: Through further separation and purification under the guidance of activity tracking, a strain with high yield and easy purification to the compound was obtained, and the strain was named L38.
[0028] The specific isolation method is as follows: firstly, the surface of the collected sporocarp is sterilized with disinfectant alcohol, and then washed with sterile water; then, the packet is cut into small pieces; finally, it is inoculated on PDA medium, and the bacterial strai...
Embodiment 2
[0036] Example 2. Production of 11-O-acetylcyathatriol and 15-O-acetylcyathatriol by Cyathus africanus L38
[0037] 1. Strain activation, fermentation culture
[0038] 1. Obtaining the seed solution:
[0039] Inoculate African black egg nest (Cyathus africanus) L38 on the slant of PDA test tube agar medium for pre-cultivation; the pre-cultivation conditions are: 25°C, dark culture for 7 days (PDA medium composition: 200g of potatoes, 20g of glucose, Agar powder 16g, dilute to 1L with purified water (pH natural)). After the mycelia covered the entire slope, the mycelium was transferred to a liquid medium for cultivation under sterile conditions to obtain a seed culture solution; the conditions for liquid cultivation were: 25° C., protected from light for 7 days. Liquid medium: glucose 4.0g / L, malt extract (Malt Extract) 10.0g / L, yeast (Yeast Extract) 4.0g / L, water to 1L; yeast extract (Yeast Extract) was purchased from Oxoid Ltd. , lot number 1074139.
[0040] 2. Solid ferm...
Embodiment 3
[0055] Anti-tumor cell proliferation activity test of the compound shown in embodiment 3, formula I
[0056] Human liver cancer cell HepG2 (ATCC HB-8065), human prostate cancer cell PC3 (ATCC CRL-1435), human lung cancer cell A549 (ATCC CCL-185), human colorectal cancer cell HCT-15 (ATCC CCL-225 ), human Cervical cancer cells Hela (ATCC CCL-2), human leukemia K562 cells, human gastric cancer cells SGC-7901 (ATCCSGC-7901), and human breast cancer cells MCF-7 (ATCC HCC1428) were purchased from the Animal Laboratory of Zhongshan Medical University.
[0057] Human liver cancer HepG2 cells, human prostate cancer PC3 cells were cultured in DMEM medium containing 10% (volume percentage) fetal bovine serum, human lung cancer A549 cells, human colorectal cancer cells HCT-15, human cervical cancer Hela cells, human Leukemia K562 cells, human gastric cancer SGC-7901 cells, and human breast cancer cells MCF-7 were cultured with RPMI 1640 medium containing 10% (volume percentage) fetal bov...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com