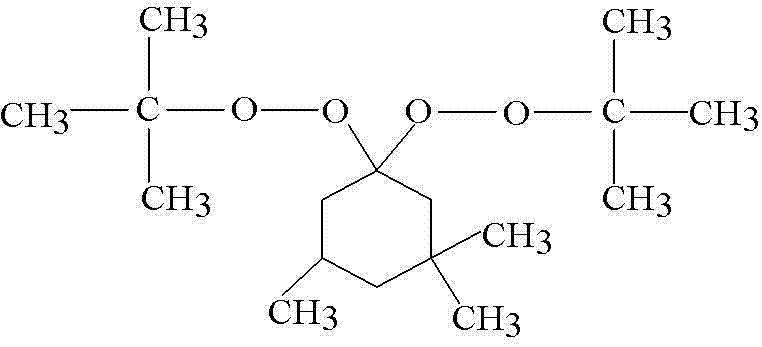
Preparation method of 1, 1-bis(t-butyl peroxy)-3, 3, 5-trimethylcyclohexane
A technology of trimethylcyclohexane and tert-butyl peroxy, which is applied in the field of 1, can solve the problems of high processing cost, high energy consumption, complicated processing technology, etc., to avoid waste liquid discharge, reduce preparation cost, protect environmental effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
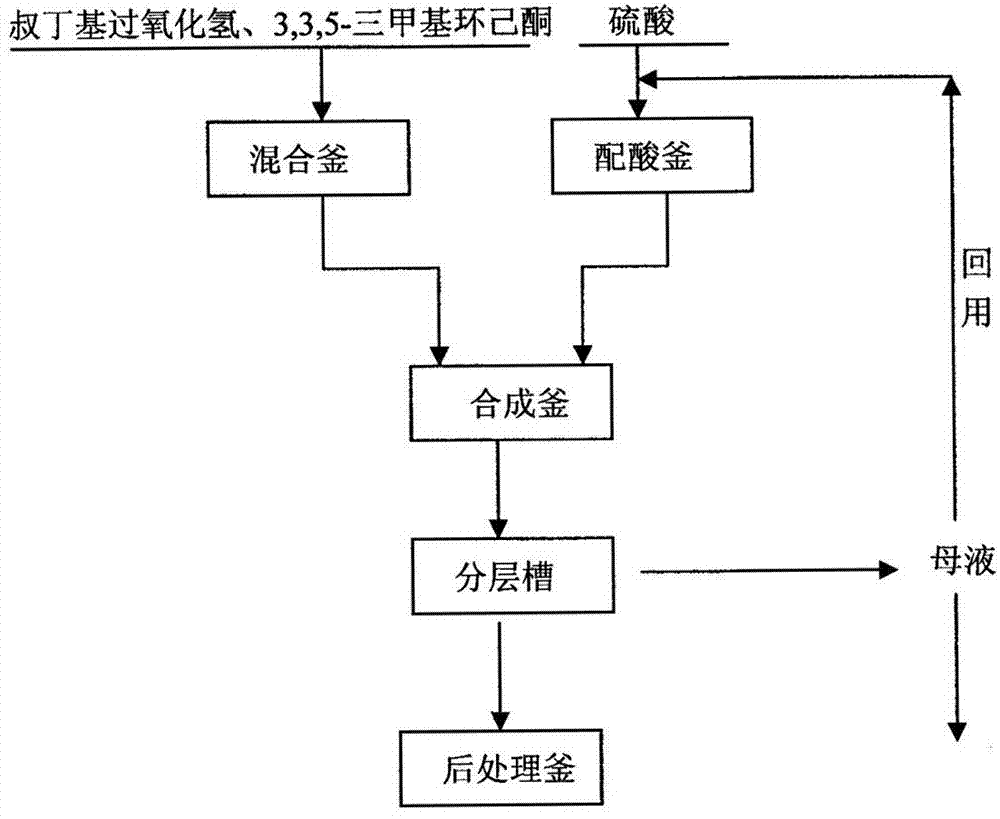
[0023] according to figure 1 The steps included in the process flow shown are as follows:
[0024] A) Add 613.9Kg tert-butyl hydroperoxide (85% by mass percentage) and 400Kg 3,3,5-trimethylcyclohexanone (99% by mass percentage) into the mixing tank, and control the temperature at Below 5°C, mix at a stirring speed of 120r / min for 10min, and after standing for 60min, separate the lower aqueous phase of 39.91Kg to obtain a mixture of tert-butyl hydroperoxide and 3,3,5-trimethylcyclohexanone Liquid 974.09Kg;
[0025] B) Add 114.78Kg of sulfuric acid (mass percentage concentration of 98%) and 885.22Kg of reused mother liquor (containing sulfuric acid mass percentage concentration of 55.1%) into the acid mixing tank, control the temperature below 5°C, and stir at 120r / min Mix 30min under condition, obtain the sulfuric acid solution 1000Kg that catalysis is used;
[0026] C) Add the mixture of 974.09Kg tert-butyl hydroperoxide and 3,3,5-trimethylcyclohexanone, and 1000Kg catalyti...
Embodiment 2
[0030] A) Add 700Kg tert-butyl hydroperoxide (mass percentage concentration: 80%) and 400Kg 3,3,5-trimethylcyclohexanone (mass percentage concentration: 99%) into the mixing tank, and control the temperature at 10 Below ℃, the stirring speed is 80r / min and mixed for 20min. After standing still for 50min, the lower aqueous phase is separated to 42.7Kg, and the mixed solution of tert-butyl hydroperoxide and 3,3,5-trimethylcyclohexanone is obtained. 1057.3Kg;
[0031] B) Add 205.08Kg of sulfuric acid (mass percentage concentration of 98%) and 654.92Kg of recycled mother liquor (containing sulfuric acid mass percentage concentration of 50.7%) into the acid mixing tank, control the temperature below 10°C, and stir at 80r / min Mix 20min under condition, obtain the sulfuric acid solution 860Kg that catalysis is used;
[0032] C) Add a mixture of 1057.3Kg tert-butyl hydroperoxide and 3,3,5-trimethylcyclohexanone, and 860Kg catalytic sulfuric acid solution into the synthesis kettle to ...
Embodiment 3
[0036] A) Add 814.6Kg tert-butyl hydroperoxide (mass percentage concentration is 75%) and 400Kg 3,3,5-trimethylcyclohexanone (mass percentage concentration is 99%) into the mixing kettle, and control the temperature at Below 15°C, mix at a stirring speed of 50r / min for 30min, and after standing still for 40min, separate out 45.62Kg of the lower aqueous phase to obtain a mixture of tert-butyl hydroperoxide and 3,3,5-trimethylcyclohexanone Liquid 1169.01Kg;
[0037] B) Add 255.96Kg of sulfuric acid (mass percentage concentration of 98%) and 474.04Kg of reused mother liquor (containing sulfuric acid mass percentage concentration of 45.6%) into the acid preparation kettle, control the temperature below 15°C, and stir at a speed of 50r / min Mix 10min under condition, obtain the sulfuric acid solution 730Kg that catalysis is used;
[0038] C) Add the mixture of 1169.01Kg tert-butyl hydroperoxide and 3,3,5-trimethylcyclohexanone, and 730Kg catalytic sulfuric acid solution into the sy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com