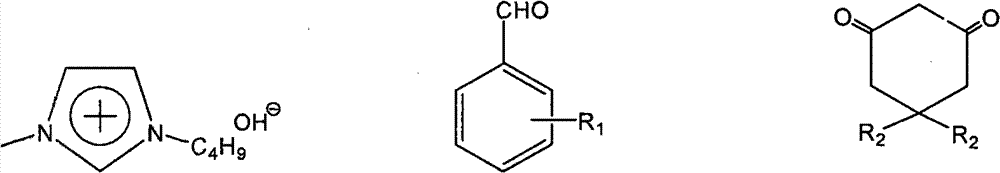
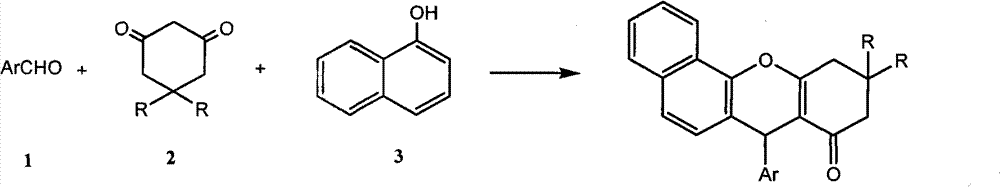
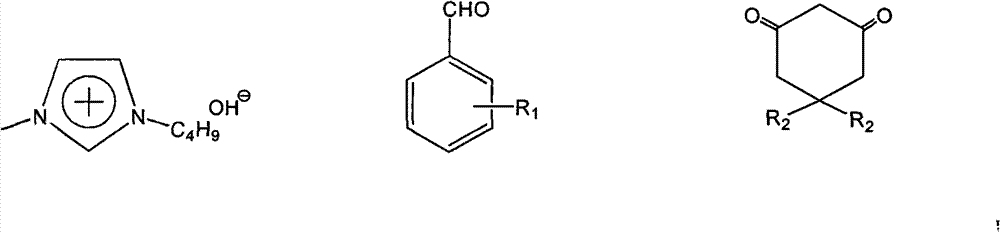
Synthesis of benzoxanthene derivatives by aqueous phase catalysis of ionic liquid
A technology of benzoxanthene and ionic liquid, which is applied in the field of synthesis of benzoxanthene derivatives catalyzed by ionic liquid in water phase, can solve the problems of lack of large-scale industrial application, catalysts that cannot be recycled, and complex catalyst preparation process, etc. problem, to achieve the effect of water stability, convenient preparation and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In a 25mL round bottom flask, add 10mmol (1.061g) benzaldehyde, 10mmol (1.121g) 1,3-cyclohexanedione, 10mmol (1.442g) α-naphthol, 0.5mmol catalyst, 4mL water in sequence, Stir and reflux at 100°C for 1 hour under normal pressure, cool, filter and wash with cold water, and recrystallize from 95% ethanol to obtain pure 7-phenyl-benzoxa[c]anthracene with a yield of 85%.
Embodiment 2
[0026] In a 25mL round bottom flask, add 10mmol (1.061g) benzaldehyde, 10mmol (1.402g) 5,5-dimethyl-1,3-cyclohexanedione, 10mmol (1.442g) α-naphthol, 0.5 mmol of catalyst, 5mL of water, mixed and stirred at 100°C under normal pressure for 1.5 hours, cooled, filtered and washed with cold water, recrystallized from 95% ethanol to obtain 10,10-dimethyl-7-phenyl-benzoxa[ c] The pure product of anthracene, the yield is 85%.
Embodiment 3
[0028] In a 25mL round bottom flask, add 10mmol (1.221g) 4-hydroxybenzaldehyde, 10mmol (1.402g) 5,5-dimethyl-1,3-cyclohexanedione, 10mmol (1.442g) α-naphthalene Phenol, 0.8mmol of catalyst, 5mL of water, mixed and stirred at 100°C under normal pressure for 2.5 hours under reflux, cooled, filtered and washed with cold water, recrystallized from 95% ethanol to obtain 10,10-dimethyl-7-(4-hydroxy- The pure product of phenyl)benzoxa[c]anthracene, the yield is 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com