DC-CIK (dendritic cells-cytokine-induced killers) cell treatment composition
A technology of cell therapy and composition, which is applied in animal cells, drug combinations, vertebrate cells, etc., can solve the problems of poor proliferation ability of CIK cells, small number of DC cells, poor antigen presentation ability, etc., and achieve high killing activity, The effect of high purity DC and strong antigen presentation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] Preparation of autologous cord blood plasma:
[0044] 1. Aseptically collect a portion of umbilical cord blood from a full-term normal fetus after umbilical cord clamping, anticoagulate with citric acid, and centrifuge in a centrifuge tube for 15 minutes.
[0045] 2. Take about 30ml of the supernatant, put it into a centrifuge tube, continue to centrifuge for 15 minutes, and collect the supernatant plasma.
[0046] 3. Put the collected plasma into a 56°C water bath for 30 minutes to inactivate complement.
[0047] 4. Centrifuge to remove the flocculent sediment in the tube, transfer the supernatant to a new centrifuge tube and freeze it for later use.
[0048] Isolation of cord blood mononuclear cells:
[0049] 1. After separating the umbilical cord blood from the upper plasma, mix it with normal saline at a ratio of 1:1, and then mix it with 6.0% (w / v) hydroxyethyl starch (HESpan) at a ratio of 6:1 , stand at room temperature for 30 minutes, wait for the erythrocyte...
Embodiment 2
[0054] Preparation of Example 2 DC
[0055] 1. Inoculate the isolated mononuclear cells into a T25 cell culture flask and store in 37°C CO 2 After overnight attachment in the incubator, the suspension cells were detached and transferred to new T25 cell culture flasks.
[0056] 2. Add 5 mL of GT-T551 medium containing 0.6% autologous cord blood plasma to the adherent cells (serum-free lymphocyte and dendritic cell medium imported from Japan TAKARA, provided by Biotech Co., Ltd.) , and add rhGM-CSF 30ng / mL, rhIL-410ng / mL, 5ng / mL SCF and 3ng / mL Flt3-L, set at 37°C 5% CO 2 Continue to grow in the incubator.
[0057] 3. Replace half the amount of medium every other day, and add complete medium containing rhGM-CSF and rhIL-4 to keep the concentration of cytokines unchanged.
[0058] 4. Add 5 μg / mL of antigenic protein extracted from the breast cancer cell line ZR-751 to stimulate on the 5th day of culture, add rhTNF-α 10 ng / mL on the 6th day, and continue to culture for 1-4 days....
Embodiment 3
[0060] Example 3 Immunophenotypic detection of DC
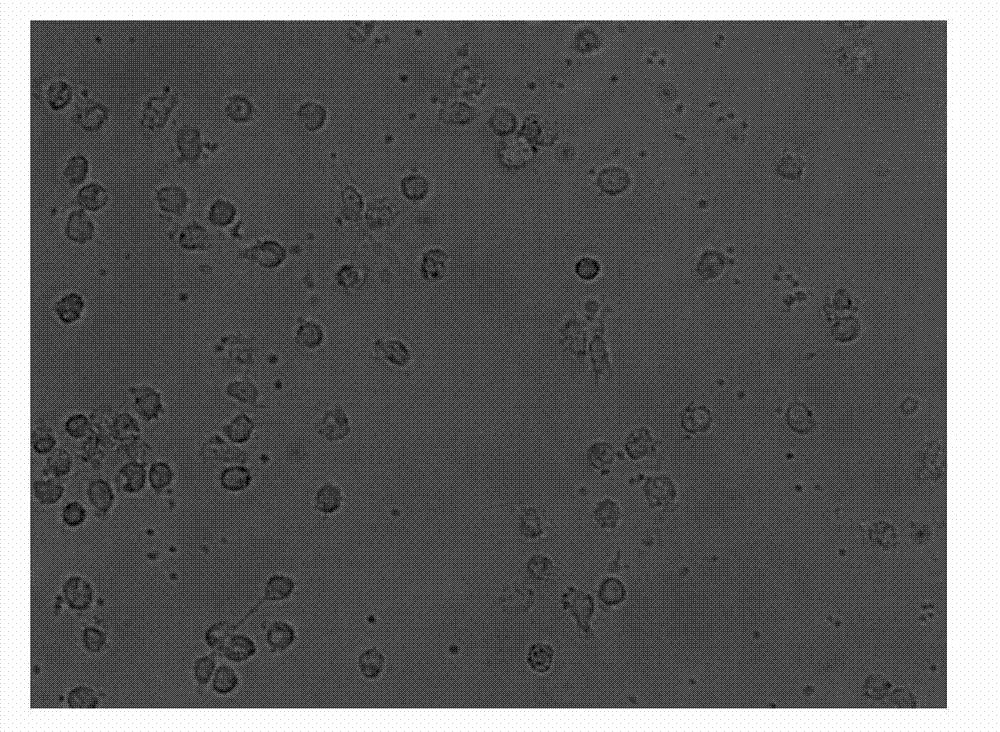
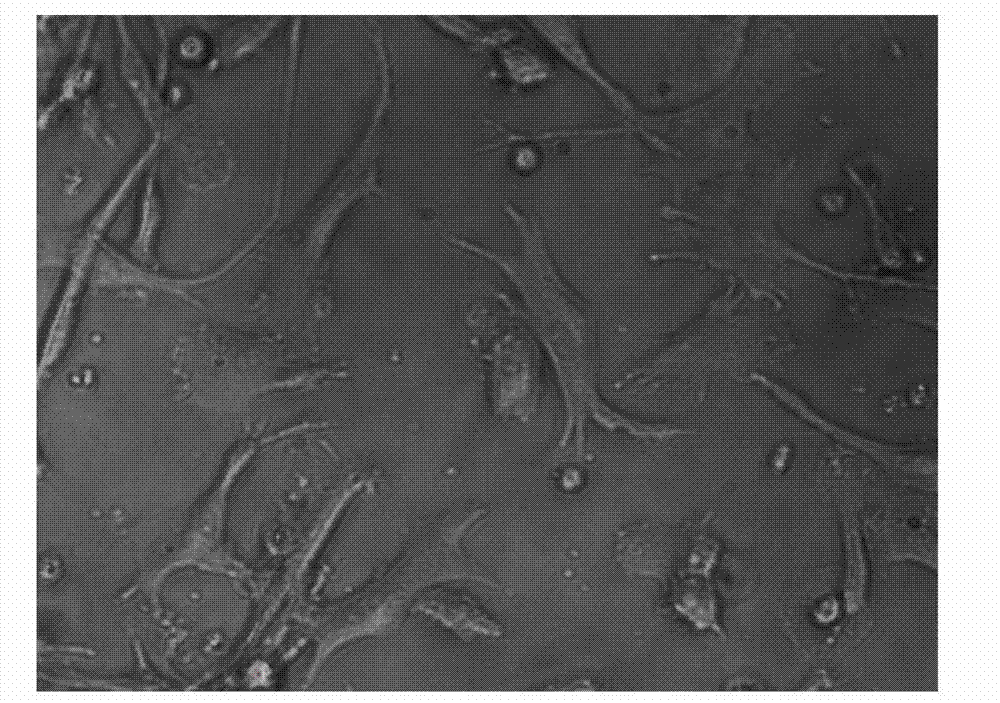
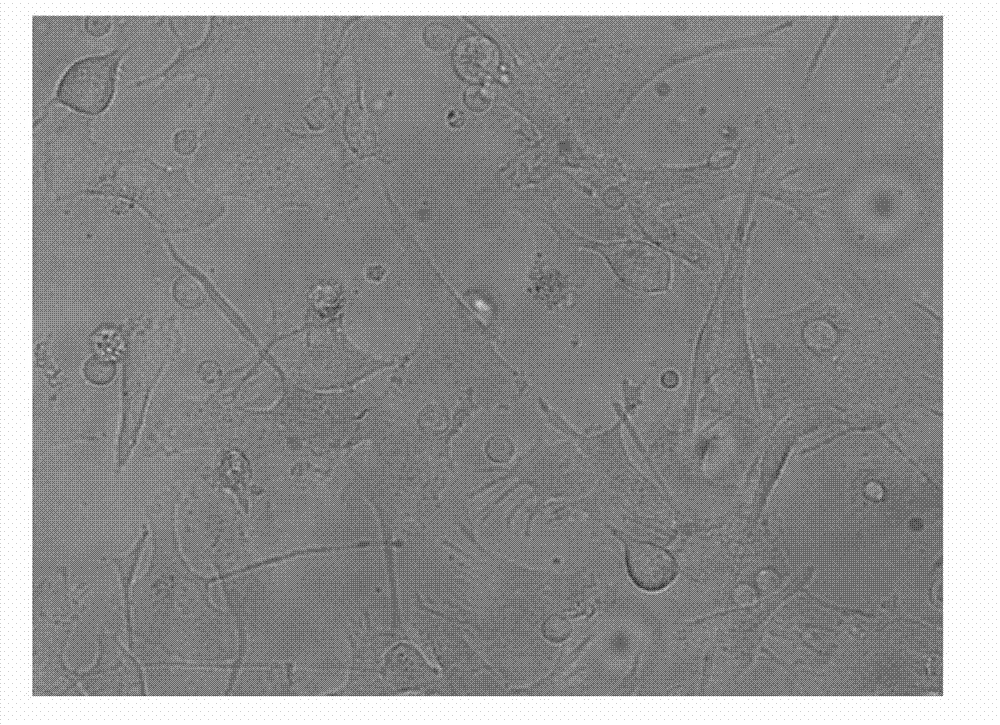
[0061] Take the cells on the 1st, 7th, and 9th day of culture respectively, wash them twice with calcium and magnesium-free PBS, and take 1×10 5 / mL were added to the corresponding FCM tubes. Add 5 μl of monoclonal antibodies to be detected, including CD1α, HLA-DR, CD80, CD83, and CD86 antibodies, incubate at 4°C in the dark for 30 minutes, and shake once every 10 minutes to fully contact the cells with the antibodies. Washed twice with PBS, resuspended in 400 μl of PBS, and detected by flow cytometer FASCSCalibur (BD Biosciences), the results are shown in Figure 2, Table 1, image 3 .
[0062] Table 1 Immunophenotype of DCs at different culture times
[0063] Training time
[0064] Among the above surface antigens, CD1α and CD80 are surface markers of DC cells, CD83 and CD86 are co-stimulatory molecules of DC cells, and HLA-DR is an immunostimulatory molecule of DC cells. It can be seen from the experimental re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com