Application of secretory type Klotho in preparing medicine for treating chronic renal failure
A chronic kidney disease, secretory technology, used in gene therapy, drug combination, urinary system diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
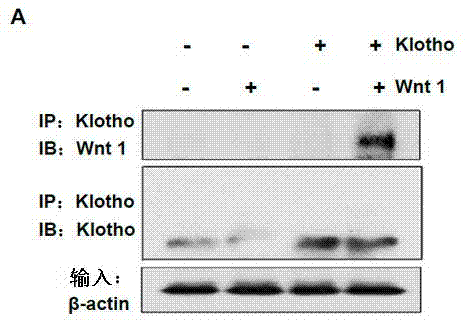
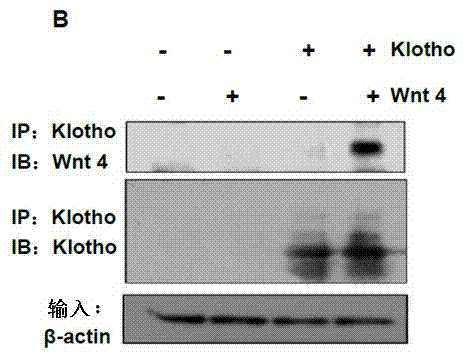
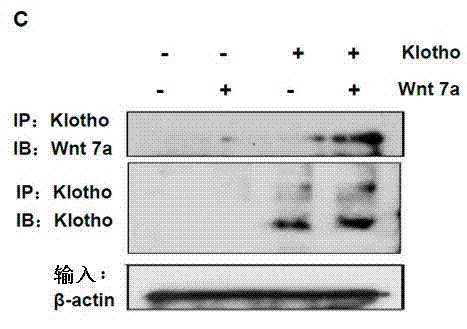
[0027] Example 1: Klotho protein binds to various Wnt proteins and inhibits Wnt / β-catenin signaling pathway activity
[0028] 1. Experimental materials:
[0029] Cells: mouse glomerular podocytes (a gift from Professor Peter Mundel, USA, see his paper Exp. Cell Res. 236: 248-258, 1997).
[0030] Differentiation medium: RPMI1640 medium containing 10% FBS, 100u / ml penicillin, and 100μg / ml streptomycin.
[0031] Proliferation culture medium: 50u / ml recombinant mouse IFN-γ was added to the above culture medium.
[0032] Proliferation culture method: cells were subcultured at 33 o C contains 5% CO 2 In the incubator, change the medium every other day.
[0033] Differentiation culture: To induce differentiation, podocytes are transferred to differentiation medium, 37 o C contains 5% CO 2 The incubator is used to differentiate podocytes for 10-14 days, and the medium is changed every other day.
[0034] 2. Experimental treatment:
[0035] Before the experiment, the podocyte...
Embodiment 2
[0039] Example 2: Inhibition of Klotho Protein on Kidney Fibrosis in UUO Mice
[0040] 1. Experimental animals: CD-1 mice, male, weighing 20-22g, SPF grade.
[0041] The animals were first weighed and numbered, and 21 healthy mice with a body weight of 20-22 g were selected and randomly divided into 3 groups, 7 in each group. Including sham operation group, model control group and medication group.
[0042] 2. Experimental grouping
[0043] 1) Sham-operated group: After the mice were anesthetized with 3% pentobarbital sodium at room temperature and 1ml / kg body weight, the incision was selected 1-2 cm below the left dorsal costal margin; after local disinfection, the skin, subcutaneous, and The muscle layer and peritoneum were sutured layer by layer after the left ureter was discovered. After local disinfection, check the marks and place them in corresponding mouse cages.
[0044] 2) Model control group: anesthetized and disinfected as above. The skin, subcutaneous layer...
Embodiment 3
[0056] Example 3: Inhibition of Klotho Protein on Kidney Fibrosis in ADR Mice
[0057] 1. Experimental animals: BABL / c mice, male, weighing 20-22g, SPF grade.
[0058] The animals were first weighed and numbered, and 21 healthy mice with a body weight of 20-22 g were selected and randomly divided into 3 groups, 7 in each group. Including normal saline group, model control group and medication group.
[0059] 2. Experimental groups
[0060] 1) Normal saline group: inject 2ml of normal saline into the tail vein at room temperature.
[0061] 2) Model control group: Doxorubicin was dissolved in normal saline, and 10 mg / kg body weight was injected into the tail vein once.
[0062] 3) Medication group: 1 week after doxorubicin injection, 2 ml of normal saline containing 1 mg / kg body weight of Klotho plasmid (same source as in Example 1) was injected into the tail vein, and then injected again every other week, for a total of 2 injections.
[0063] 3. Experimental procedure
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com