Mupirocin purification method
A mupirocin and purification method technology, which is applied in the field of mupirocin purification, can solve the problems of large amount of organic solvent usage, many extraction steps, and low purity, and achieve energy saving, production cost reduction, and excellent product quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
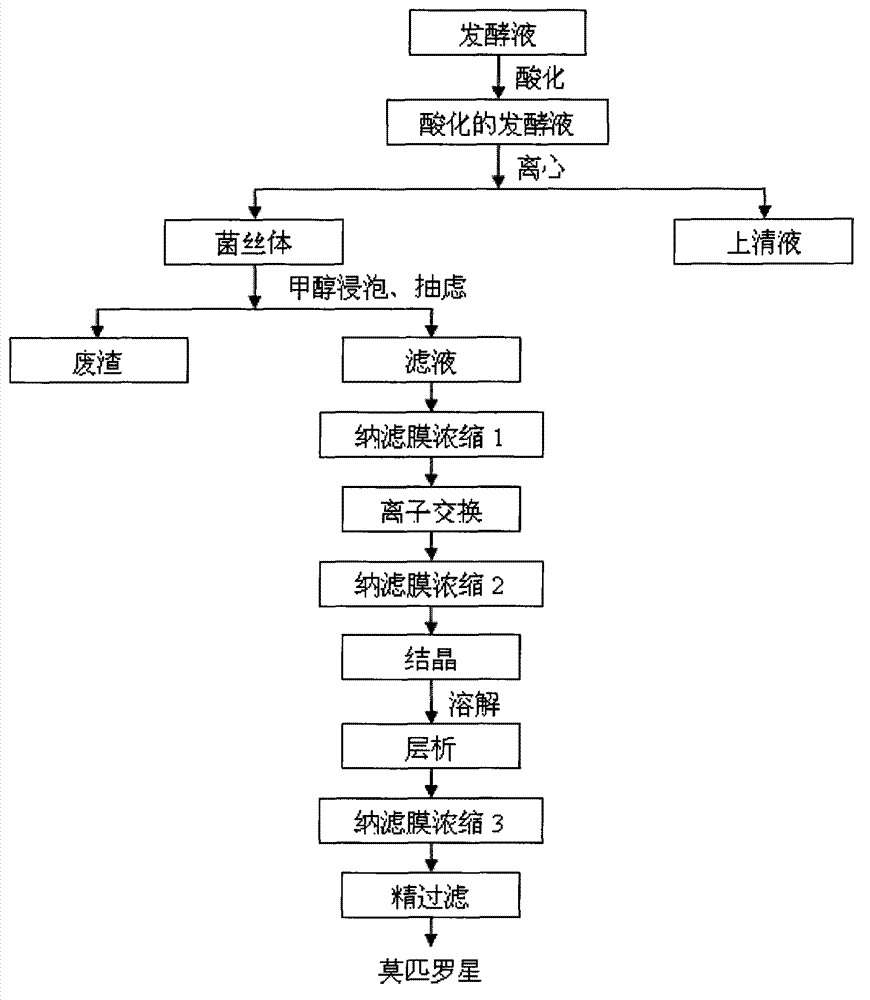
[0036] Measure 100L of fermentation broth, add 1M hydrochloric acid, adjust the pH value to 4.0, stir for 20 minutes, and centrifuge at 3000r / min to obtain mycelium, which is set aside. Add 2 times the volume of methanol to the obtained mycelium after the acidification treatment, stir evenly, soak for 4 hours, and keep the filtrate for future use after suction filtration.
[0037] Select the nanofiltration membrane with a cut-off molecular weight of 500-600 for the first nanofiltration concentration, set the inlet and outlet temperatures at room temperature, and filter the organic solvent to obtain the filtrate with a feed rate of 1.0L / h and an operating pressure of 0.5MPa. Concentrate by nanofiltration, set aside.
[0038] Add 5% polystyrene divinylbenzenesulfonate DOW[XFS.43278.002] sodium strong acid resin to the nanofiltration concentrate, stir at room temperature for 6 hours, rinse the resin with deionized water, and then use a sodium hydroxide solution with a pH of 10.0 ...
Embodiment 2
[0043] Measure 100L of fermentation broth, add 1M hydrochloric acid, adjust the pH value to 4.5, stir for 20min, and centrifuge at 3000r / min to obtain mycelium, which is set aside. Add 2 times the volume of methanol to the obtained mycelium after the acidification treatment, stir evenly, soak for 4 hours, and keep the filtrate for future use after suction filtration.
[0044] Select the nanofiltration membrane with a cut-off molecular weight of 500-600 for the first nanofiltration concentration, set the inlet and outlet temperatures at room temperature, and filter the organic solvent to obtain the filtrate with a feed rate of 1.2L / h and an operating pressure of 0.6MPa. Concentrate by nanofiltration, set aside.
[0045] Add 5% polystyrene divinylbenzenesulfonate DOW[XFS.43278.002] sodium strong acid resin to the nanofiltration concentrate, stir at room temperature for 8 hours, rinse the resin with deionized water, and then use a sodium hydroxide solution with a pH of 10.5 Deso...
Embodiment 3
[0050] Measure 100L of fermentation broth, add 1M acetic acid, adjust the pH value to 4.2, stir for 20min, and centrifuge at 3000r / min to obtain mycelium, which is set aside. Add 2 times the volume of methanol to the obtained mycelium after the acidification treatment, stir evenly, soak for 4 hours, and keep the filtrate for future use after suction filtration.
[0051] Select the nanofiltration membrane with a cut-off molecular weight of 500-600 for the first nanofiltration concentration, set the inlet and outlet temperatures at room temperature, and filter the organic solvent to obtain the filtrate with a feed rate of 1.0L / h and an operating pressure of 0.4MPa. Concentrate by nanofiltration, set aside.
[0052] Add 5% polystyrene divinylbenzenesulfonate DOW[XFS.43278.002] sodium strong acid resin to the nanofiltration concentrate, stir at room temperature for 7 hours, rinse the resin with deionized water, and then use a sodium hydroxide solution with a pH of 10.0 Desorb, co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com