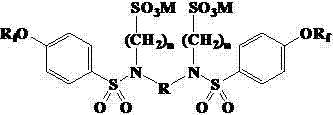Sulfonic acid dimeric surfactant based on perfluoroolefine and preparation method thereof
A gemini surface, perfluoroolefin technology, applied in the preparation of sulfonic acid amides, chemical instruments and methods, organic chemistry, etc., can solve the problems of insufficient surface activity of fluorosurfactants, achieve high decontamination and washing ability, reduce surface Tension, good dispersion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
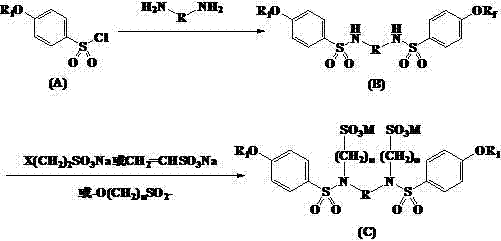
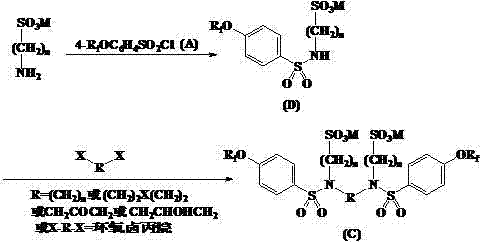
[0027] Example 1 Condensation of perfluorononenyloxybenzenesulfonyl chloride and ethylenediamine
[0028] A solution of 12.60g (20.2mmol) of perfluorononenyloxybenzenesulfonyl chloride in 20mL of N,N-dimethylformamide was slowly added dropwise to 0.60g (10.0mmol) of ethylenediamine and sodium hydroxide at 45°C In a mixture of 0.82g (20.5mmol) and 25mL of water, drop it over in about 30min, keep the reaction at 60°C for 12h, cool, filter, wash with water, and dry in vacuo to obtain 11.8g of light yellow solid with a yield of 95.8%.
Embodiment 2
[0029] Example 2 Condensation of perfluorohexenyloxybenzenesulfonyl chloride and 1,4-butanediamine
[0030] A solution of 9.55g (20.2mmol) of perfluorohexenyloxybenzenesulfonyl chloride in 20mL of acetonitrile was slowly added dropwise to 0.88g (10.0mmol) of 1,4-butanediamine, 1.45g (10.5mmol) of potassium carbonate at 45°C ) and 25mL of water, dripped in about 30min, kept at 60°C for 18h, cooled, filtered, washed with water, and dried in vacuo to obtain 9.0g of light yellow solid with a yield of 93.7%.
Embodiment 3
[0031] Example 3 Condensation of N,N'-bis(perfluorononenyloxybenzenesulfonyl)ethylenediamine and sodium chloroethylsulfonate
[0032] N,N'-bis(perfluorononenyloxybenzenesulfonyl)ethylenediamine 6.16g (5.0mmol), sodium chloroethylsulfonate 3.33g (20.0mmol) and water 25mL were mixed, and sodium carbonate was added to adjust the pH to 10, react at 60°C for 24 hours, and add sodium carbonate during the reaction to keep the pH basically unchanged. Cool, filter, wash, and dry in vacuo to obtain 6.3 g of an amber solid product with a yield of 84.4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface tension | aaaaa | aaaaa |
| Minimum surface tension | aaaaa | aaaaa |
| Minimum surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com