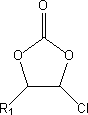
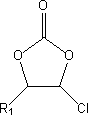
Method for preparing fluoro-carbonate ester by means of phase-transfer catalysis
A technology of phase transfer catalysis and fluorocarbonate, which is applied in the direction of organic chemistry, can solve the problems of high tar, low yield, low conversion rate and selectivity, and achieve easy-to-use, high reaction yield and stable catalyst Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 700g of chloroethylene carbonate and 1200g of dimethyl carbonate to a 3000ml four-necked round bottom flask equipped with mechanical stirring, thermometer and reflux condenser, stir and mix well, then add 398g of solid KF and 18g of p-tert-butyl cup [4] Aromatics. Heating in an oil bath, the temperature was raised to 70° C. for 8 hours. The gas chromatography monitoring reaction result shows that 97.3% of chloroethylene carbonate reacts, the selectivity of fluoroethylene carbonate is 92.8%, and the yield of target product fluoroethylene carbonate reaches 90.3%.
Embodiment 2
[0029] Add 750g of chloroethylene carbonate and 1300g of diethyl carbonate to a 3000ml four-necked round-bottomed flask equipped with mechanical stirring, thermometer and reflux condenser. After mixing evenly, add 427g of solid KF and 29g of p-tert-butyl cup [6] Aromatics. Heated in an oil bath, and the temperature was raised to 110°C for 10 hours of reaction. The gas chromatography monitoring reaction results showed that 99.3% of the chloroethylene carbonate reacted, the selectivity of the fluorination reaction was 95.9%, and the yield of the target product fluoroethylene carbonate reached 95.2%.
Embodiment 3
[0031] In the four-neck round bottom flask that 2000ml is furnished with mechanical stirring, thermometer, reflux condenser, add 550g 4-chloro-5-methyl-1,3-dioxolan-2-ketone and 800g diethyl carbonate, After stirring and mixing, 315g of solid KF and 25g of p-hydrocalix[6]arene were added. Heated in an oil bath, and the temperature was raised to 100°C for 7 hours of reaction. The gas chromatography monitoring reaction result shows that 98.6% of 4-chloro-5-methyl-1,3-dioxolan-2-ketone reacts, and the selectivity of fluorination reaction is 96.2%, and target product 4-fluoro- The yield of 5-methyl-1,3-dioxolan-2-one reached 94.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com