System and method for quantitatively detecting impurities in sulfur hexafluoride gas
A technology for quantitative detection of sulfur hexafluoride gas is applied in a system for quantitatively detecting impurities in sulfur hexafluoride gas, and in the field of quantitative detection of impurities in sulfur hexafluoride gas, which can solve problems such as those not mentioned, and achieve rapid detection, High sensitivity, good response linearity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
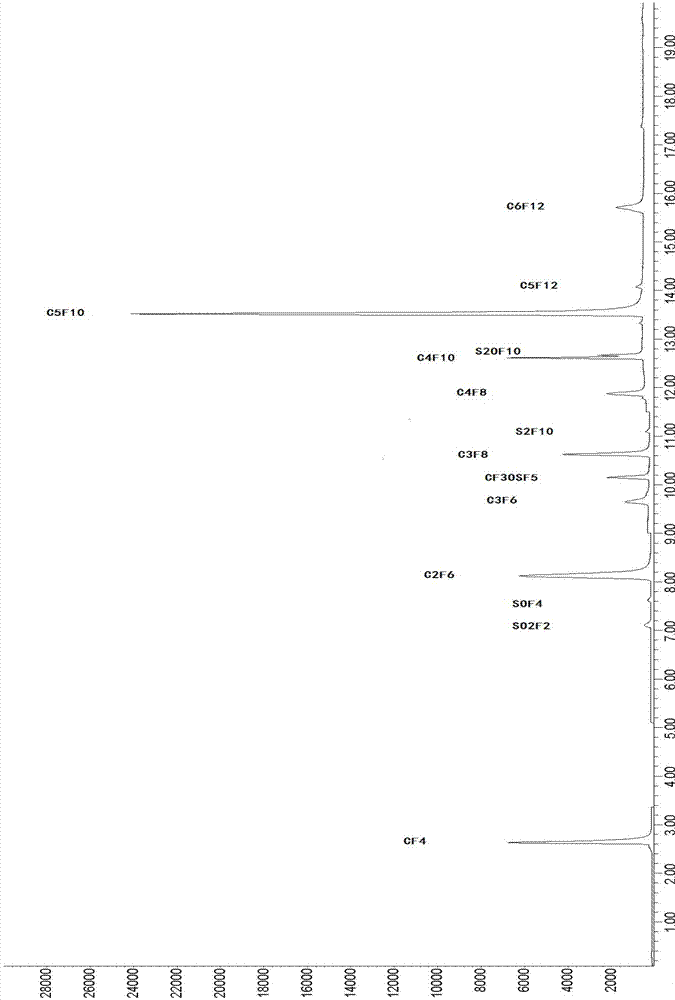
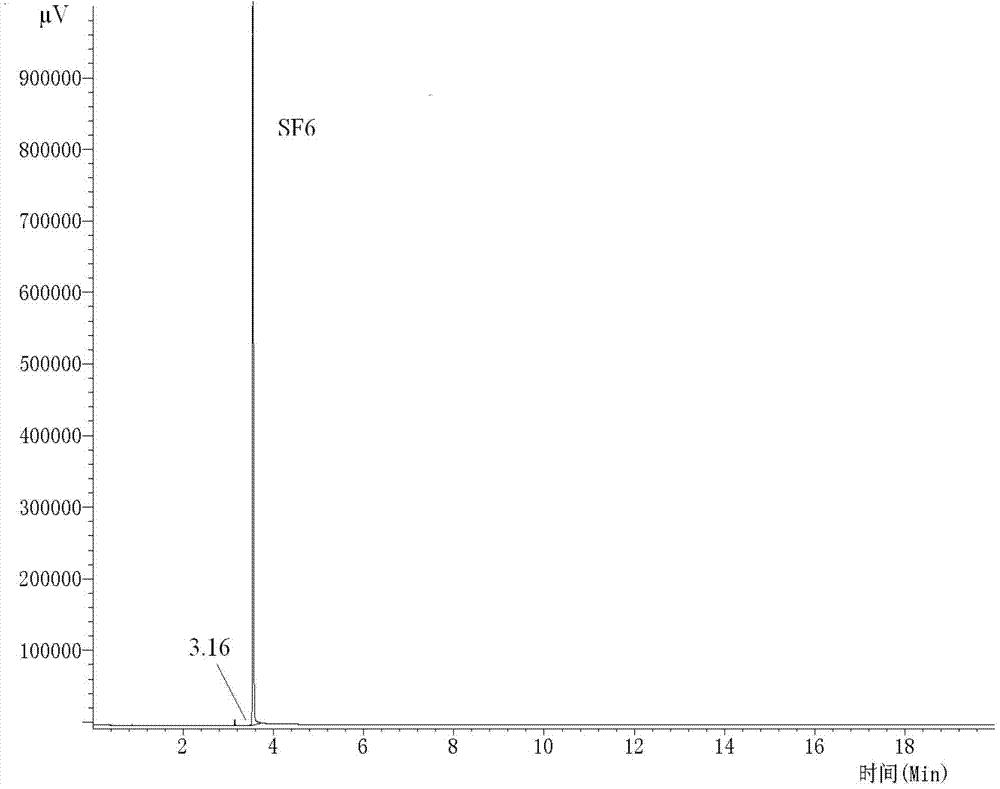
[0048] This embodiment uses the detection system and method of the present invention to detect SF 6 Cylinder gas, quantitative analysis of air, fluorocarbon and sulfur-containing fluoride impurities.
[0049] 1. Detection system
[0050] GC-MS model: Agilent 6890-5973N.
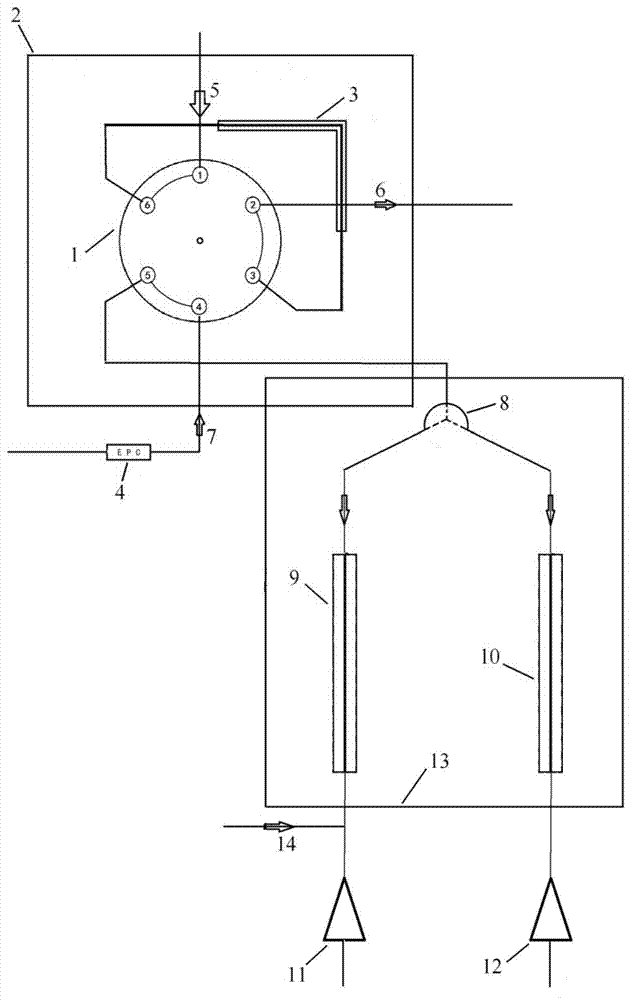
[0051] Equipped with double-column, double-detector parallel device: injector six-way valve 1 (the through-way valve and its deactivation pipeline are placed in a valve box 2; the six-way valve is connected with the carrier gas flow path and the exhaust port, and the load There is also an electronic flow controller 4 in the gas flow path, and a quantitative loop 3) between the inlet 5 and the exhaust port 6 of the six-way valve, and an adjustable outlet splitter 8 is connected to it, and the flow from the adjustable outlet is divided. There are two paths separated by 8 at the device, and one path is the chromatographic column HP-PLOT Al connected in series in sequence. 2 o 3 M 10 and mass spectrometry det...
Embodiment 2
[0073] In this example, high-purity SF with a known air content of 49ppm (predetermined in accordance with GB / T12022-2006 "Industrial Sulfur Hexafluoride") is used in the laboratory. 6 The experimental gas is used as a standard gas, and the system and method of the present invention (conditions are the same as in Example 1) are used to detect standard SF 6 The air content in the gas is then determined in the method of the invention relative to the SF of the air 6 The correction factor for the gas.
[0074] Carry out three consecutive sampling analysis, and use the following formula to convert the relative SF of air 6 Gas correction factor: ωi=100fiAi / ∑(fiAi)(i=1,2,3…)
[0075] ωi——volume percent concentration of component i
[0076] fi——correction factor of component i
[0077] Ai——peak area of component i
[0078] Specify SF 6 A correction factor of 1 yields the relative SF of air 6 The correction factor is 0.43, see the table below for details:
[0079]
Embodiment 3
[0081] The present embodiment adopts that the air content in the laboratory is 334ppm, C 3 f 8 The content is 11.20ppm, SO 2 f 2 The content is 6.73ppm, S 2 f 10 The SF6 experimental gas with an O content of 9.31ppm is used as the standard experimental gas (collect pure impurity gas by laboratory means, and then add it to high-purity sulfur hexafluoride gas, so that the standard sulfur hexafluoride gas with known impurity content is obtained ), with the system and method of the present invention (conditions are the same as in Example 1) to detect standard SF 6 Air in gases, fluorocarbons and sulfur-containing fluorides. Considering that it is difficult to use highly toxic pure samples as quantitative standard samples in actual analysis and testing, we use the pressure gas distribution method to use helium to test the laboratory standard SF 6 The experimental gas is accurately diluted, and the dilution ratios (V / V) are 0, 2, 5, 10, and 20 times respectively, and the quant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com