Application of astaxanthin in fetal alcohol spectrum disorders (FASD)
A technology related to astaxanthin, applied in the direction of medical preparations containing active ingredients, organic active ingredients, non-central analgesics, etc., to achieve good prevention, improve drug utilization, and reduce pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
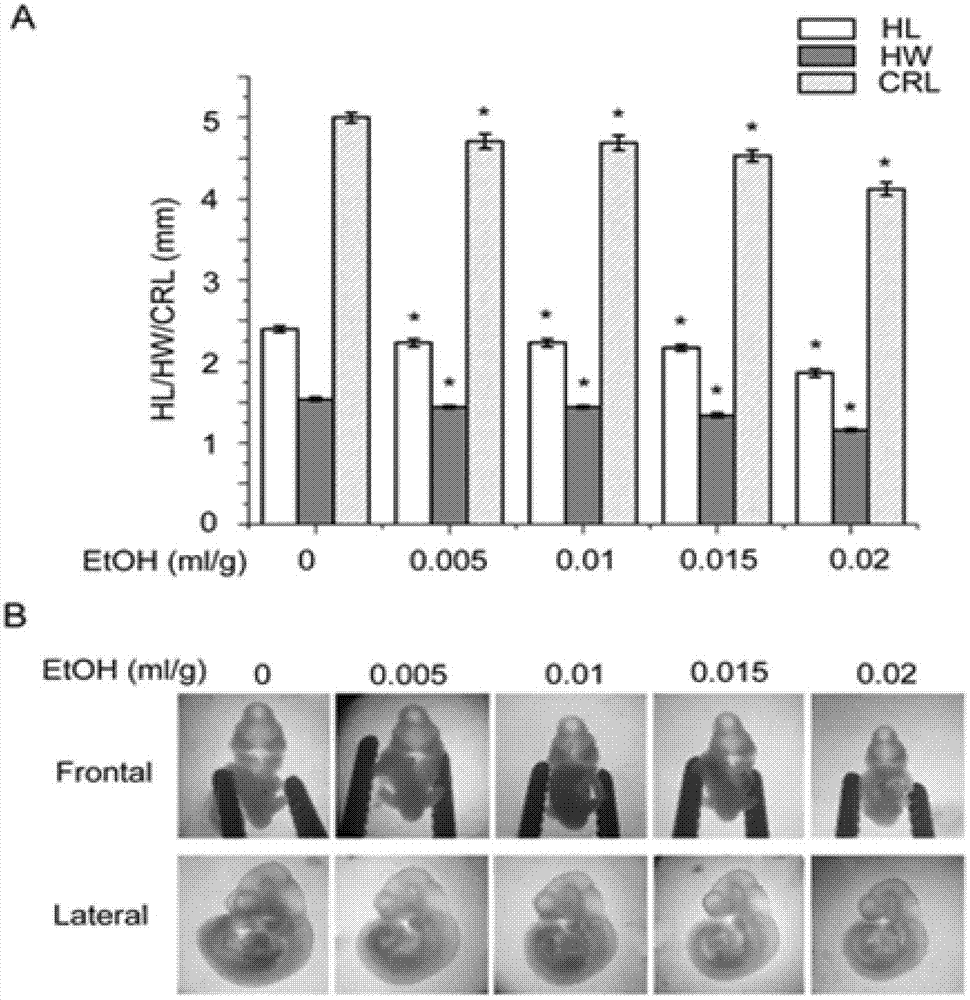
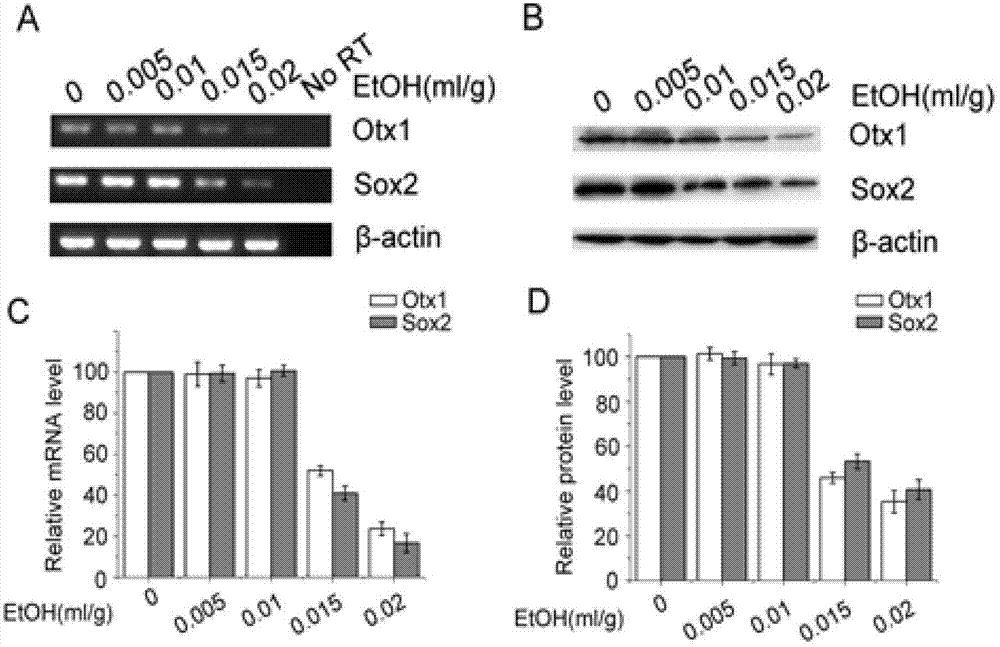
[0035] Embodiment 1 Astaxanthin inhibits the influence of alcohol on embryonic development
[0036] Inject 0, 0.005, 0.01, 0.015, 0.02ml / g of 25% ethanol intraperitoneally into C57BL / 6J mice on day 8 of pregnancy (G8), 3 pregnant mice in each group, and take out the embryos under a dissecting microscope at G10.25 Dissect and observe its shape, measure head length (HL), head width (HW) and head-rump length (CRL); in addition, C57BL / 6J mice on day 8 of pregnancy (G8) were intraperitoneally injected with 0, 0.005, 0.01, 0.015, 0.02ml / g 25% ethanol, 3 pregnant mice in each group, the embryos were taken out at G9.25 and the brain tissue was taken for RT-PCR and Western blot detection.
[0037]1 μg of total RNA was reverse-transcribed into cDNA at 37°C for 15 minutes and 85°C for 5 seconds. cDNA was subjected to polymerase chain reaction PCR with Premix Taq. The primers for Otx1 are: sense: 5'GCAGAGCGGGAATGGAAC3' (SEQ ID NO: 1), antisense: 5'AGATGGACGAAGCAGTAGGC3' (SEQ ID NO: 2) (...
Embodiment 2
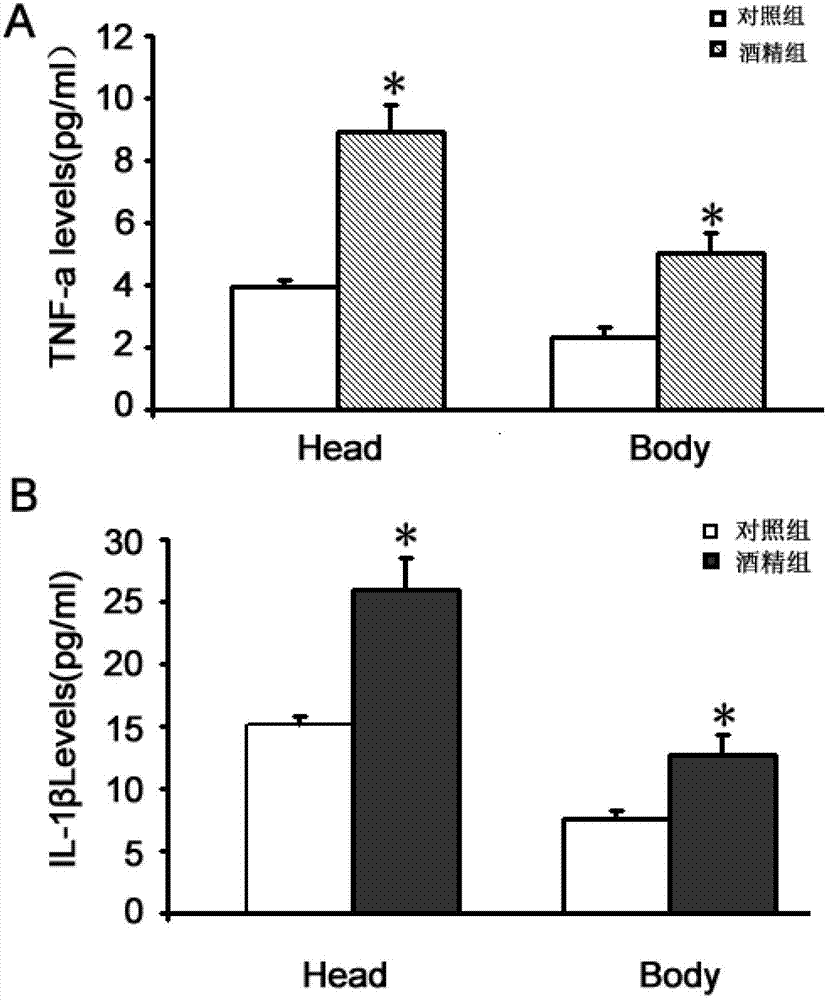
[0040] Example 2 The preventive effect of astaxanthin on fetal alcohol-related diseases
[0041] Thirty-five pregnant mice were randomly divided into 7 groups, 5 in each group, and 4 groups were intraperitoneally injected with 25% ethanol (dissolved in lactate Ringer's injection (LR), v / v) at 10:30 on G8. 0.02ml / g, and intraperitoneally inject AST (0.5, 5, 25 and 50mg / kg, dissolved in NS) at 10:00 of G7 and G8, which is equivalent to a daily dose of 1.0, 10, 50, 100mg / kg·d. In the alcohol control group, 25% ethanol 0.02ml / g was injected intraperitoneally at 10:30 in G8, and NS 0.02ml / kg was injected intraperitoneally at 10:00 in G7 and G8. The drug control group was intraperitoneally injected with LR 0.02ml / g at 10:30 of G8, and AST (50mg / kg) was injected intraperitoneally at 10:00 of G7 and G8, which is equivalent to a total daily dose of 100mg / kg·d . The normal control group was intraperitoneally injected with LR 0.02ml / g. as follows:
[0042] (1) LR (0.02ml / g for G8) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com