Method for preparing halogenated aniline from halogenated nitrobenzene by catalytic hydrogenation
A halogenated nitrobenzene, catalytic hydrogenation technology, applied in the preparation of amino compounds, preparation of amino hydroxy compounds, chemical instruments and methods, etc., can solve the problems of increasing the difficulty of recycling precious metal catalysts, and the complex preparation process of modified catalysts. , to achieve the effect of obvious inhibition of dehalogenation, reduction of separation and purification, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 5
[0029] Examples 1 to 5 investigated the performance of Pd / C catalysts treated with different sulfides in the reaction of catalytic hydrogenation to prepare haloarylamines. Prepare 10 g of Pd / C with a load of 5 wt% and 100 ml of methanol to make a slurry at 40 °C, add 5 mmol of different sulfides, stir for 2 h; filter, and dry the filter cake in vacuum at 100 °C to obtain sulfurized Treated Pd / C catalyst.
[0030] In a 500ml stainless steel reactor, add 100 g of o-chloronitrobenzene, 200 ml of ethanol, and 0.5 g of sulfurized Pd / C catalyst, close the reactor, replace the air in the reactor with nitrogen three times, and then replace it with hydrogen three times ; Raise the temperature to 80°C and the hydrogen pressure to 2 MPa, start stirring at a stirring rate of 900 r / min, and react for 1 h; stop the reaction, and when the temperature drops to room temperature, take out the reaction liquid, filter to remove the catalyst, and use gas chromatography for the filtrate analyze. ...
Embodiment 6 10
[0034] Embodiment six to ten have investigated the Pt / Al after different vulcanization condition treatment 2 o 3 Catalyst performance in catalytic hydrogenation to prepare haloarylamines. 10 g of Pt / Al with a loading of 3 wt% 2 o 3 Prepare a slurry with 100 ml of ethanol, add 1 mmol of diphenyl sulfide, stir for several hours; filter, and vacuum-dry the filter cake at 100 °C to obtain sulfurized Pt / Al 2 o 3 catalyst.
[0035] In a 500ml stainless steel reaction kettle, add 100 g 3,5-dichloronitrobenzene, 200 ml ethanol, 1 g sulfurized Pt / Al 2 o 3 Catalyst, close the reactor, replace the air in the reactor with nitrogen three times, then replace it with hydrogen three times; raise the temperature to 70 ℃, hydrogen pressure is 3 MPa, start stirring, stirring rate 900 r / min, reaction 1 h; stop After the reaction, the temperature was lowered to room temperature, the reaction solution was taken out, the catalyst was removed by filtration, and the filtrate was analyzed by gas...
Embodiment 17 20
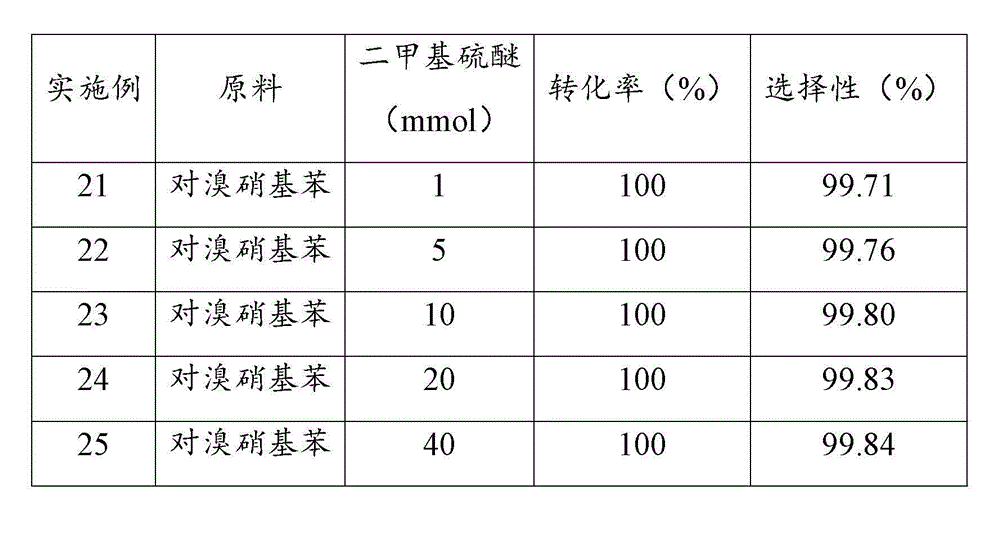
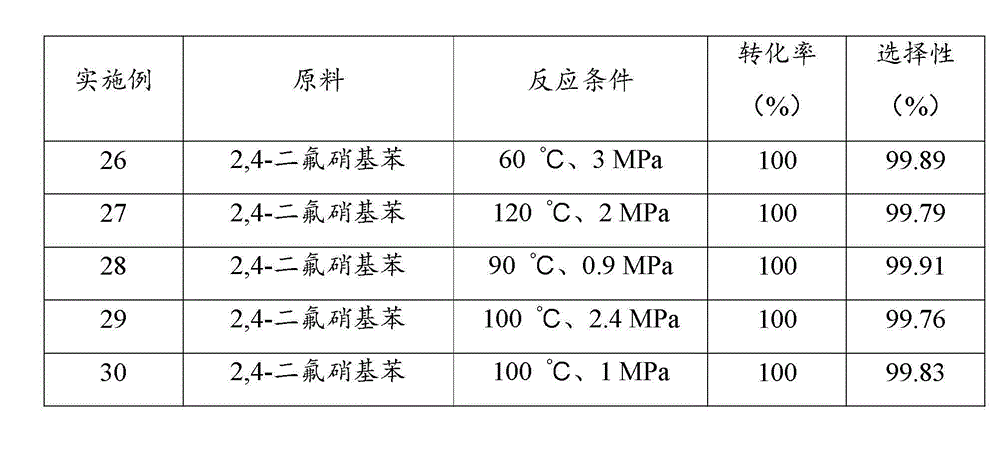
[0044] Examples 17 to 20 investigated the performance of palladium catalysts loaded on different supports after sulfidation treatment in the reaction of catalytic hydrogenation to prepare halogenated arylamines. Prepare a slurry of 10 g of supported palladium catalyst with a loading of 10 wt% and 100 ml of THF at 50 °C, add 2 mmol of dimethyl sulfide, stir for 3 h; filter, and dry the filter cake in vacuum at 100 °C, namely Obtained supported palladium catalyst through sulfide treatment.
[0045] In a 500ml stainless steel reactor, add 100 g of 6-chloro-2-nitrotoluene, 200 ml of tetrahydrofuran, 0.4 g of supported palladium catalyst through sulfide treatment, close the reactor, replace the air in the reactor with nitrogen for three times, and then Replace with hydrogen three times; raise the temperature to 110 °C, hydrogen pressure to 1.5 MPa, start stirring at a stirring rate of 900 r / min, and react for 2 h; stop the reaction, and after the temperature drops to room temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com