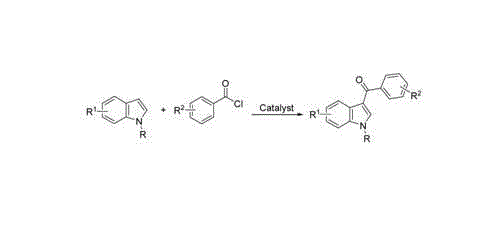
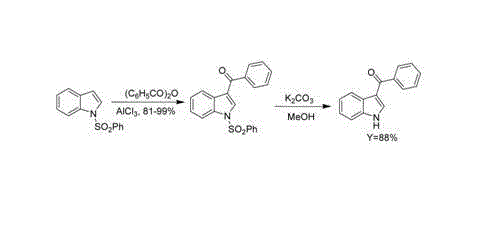
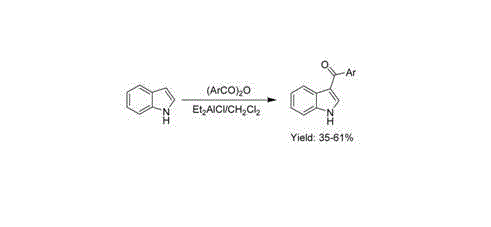
3-aroyl indole compound synthesis method
The technology of an aroyl indole compound and a synthesis method is applied in the field of synthesis of 3-aroyl indole compounds, can solve the problems of carrying out in a glove box and high reaction cost, and achieves the effects of mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Synthesis of 5-methoxy-3-benzoyl indole
[0047] 5-Methoxyindole (0.8 mmol, 117.8 mg), benzoylformic acid (1.6 mmol, 240.2 mg), copper acetate monohydrate (0.16 mmol, 31.9 mg), silver carbonate (1.6 mmol, 441.2 mg), Dimethyl sulfoxide (4 mL) was added to a 25 mL two-necked flask equipped with a magnetic stirrer, heated to 80 °C for the reaction, followed by high performance liquid chromatography or thin layer chromatography. After the raw materials were consumed, the reaction solution was cooled. to room temperature, add ethyl acetate (5 mL) and water (5 mL), transfer it to a centrifuge tube for centrifugation, then transfer the clear liquid to a separatory funnel for separation, and dichloromethane for the aqueous phase Extraction (2×10 mL), the organic phase was washed with saturated aqueous sodium bicarbonate solution (3×10 mL), the organic layer was collected, dried over anhydrous sodium sulfate, filtered, the filtrate was collected, added with silica ge...
Embodiment 2
[0049] Example 2: Synthesis of 5-methyl-3-benzoyl indole
[0050] Combine 5-methylindole (0.8 mmol, 104.9 mg), benzoylformic acid (1.6 mmol, 240.2 mg), copper acetate monohydrate (0.16 mmol, 31.9 mg), silver carbonate (1.6 mmol, 441.2 mg), dimethine Methyl sulfoxide (4 mL) was added to a 25 mL two-necked flask equipped with a magnetic stirring bar, and the experimental procedure was as in Example 1 to obtain 144.0 mg of a pale yellow solid, which was dried in vacuo, the isolated yield was 76.5%, and the melting point was 227.0- Its structure was characterized by NMR and HRMS at 228.0°C.
[0051] 1 H NMR (500 MHz, DMSO- d 6 ): δ 11.97 (s, 1H), 8.08 (1H, s), 7.87 (s, 1H), 7.77 (d, J =8.0 Hz, 2H), 7.60 (t, J =7.0 Hz, 1H), 7.53 (t, J =7.3 Hz, 2H), 7.40 (d, J =8.5 Hz, 1H), 7.09 (d, J =7.5 Hz, 1H), 2.44 (s, 3H); 13 C NMR (125 MHz, DMSO- d 6 ) δ 190.38, 141.17, 136.16, 135.52, 131.38, 131.19, 128.81, 128.77, 127.01, 125.06, 121.69, 115.16, 112.33, 21.82. HRMS (ESI) calcd...
Embodiment 3
[0052] Example 3: Synthesis of 6-methoxy-3-benzoyl indole
[0053]6-Methoxyindole (0.8 mmol, 117.8 mg), benzoylformic acid (1.6 mmol, 240.2 mg), copper acetate monohydrate (0.16 mmol, 31.9 mg), silver carbonate (1.6 mmol, 441.2 mg), Dimethyl sulfoxide (4 mL) was added to a 25 mL two-necked flask equipped with a magnetic stirring bar, and the experimental procedure was as in Example 1 to obtain 74.4 mg of a pale yellow solid, which was dried in vacuo, the isolated yield was 37.0%, and the melting point was 226.0 -227.5℃, the structure was characterized by NMR and HRMS.
[0054] 1 H NMR (500 MHz, DMSO- d 6 ): δ 11.87 (s, 1H), 8.11 (d, J =9.0 Hz, 1H), 7.80 (s, 1H), 7.87 (s, 1H), 7.77 (d, J =7.0 Hz, 2H), 7.60 (t, J =7.0 Hz, 1H), 7.53 (t, J =7.0 Hz, 2H), 7.00 (d, J =8.0 Hz, 1H), 3.80 (s, 3H); 13 C NMR (125 MHz, DMSO- d 6 ) δ 190.28, 157.07, 141.03, 138.11, 135.26, 131.42, 128.81, 128.77, 122.59, 120.71, 115.60, 112.13, 95.77, 55.75. HRMS (ESI) calcd for C 16 H 13 NO ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com