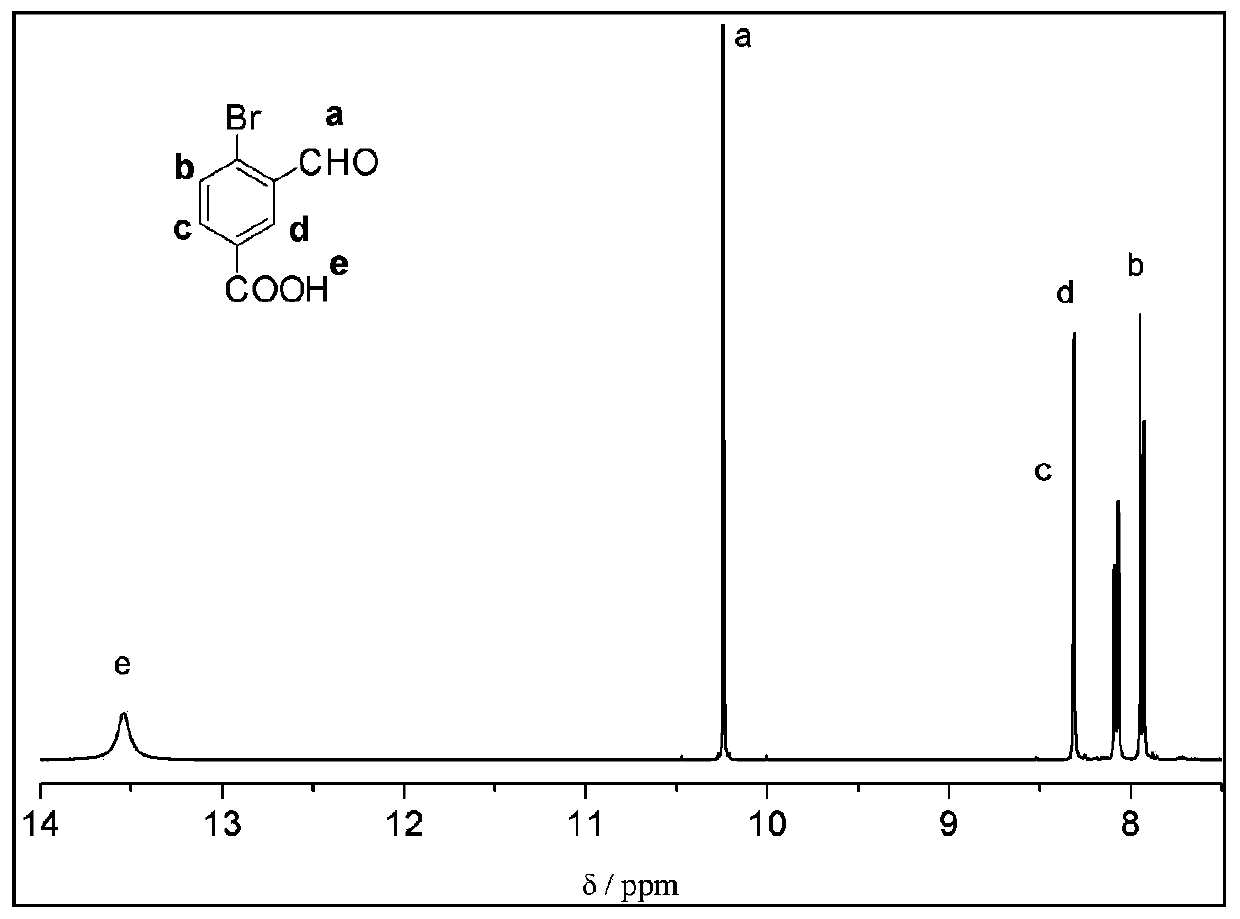
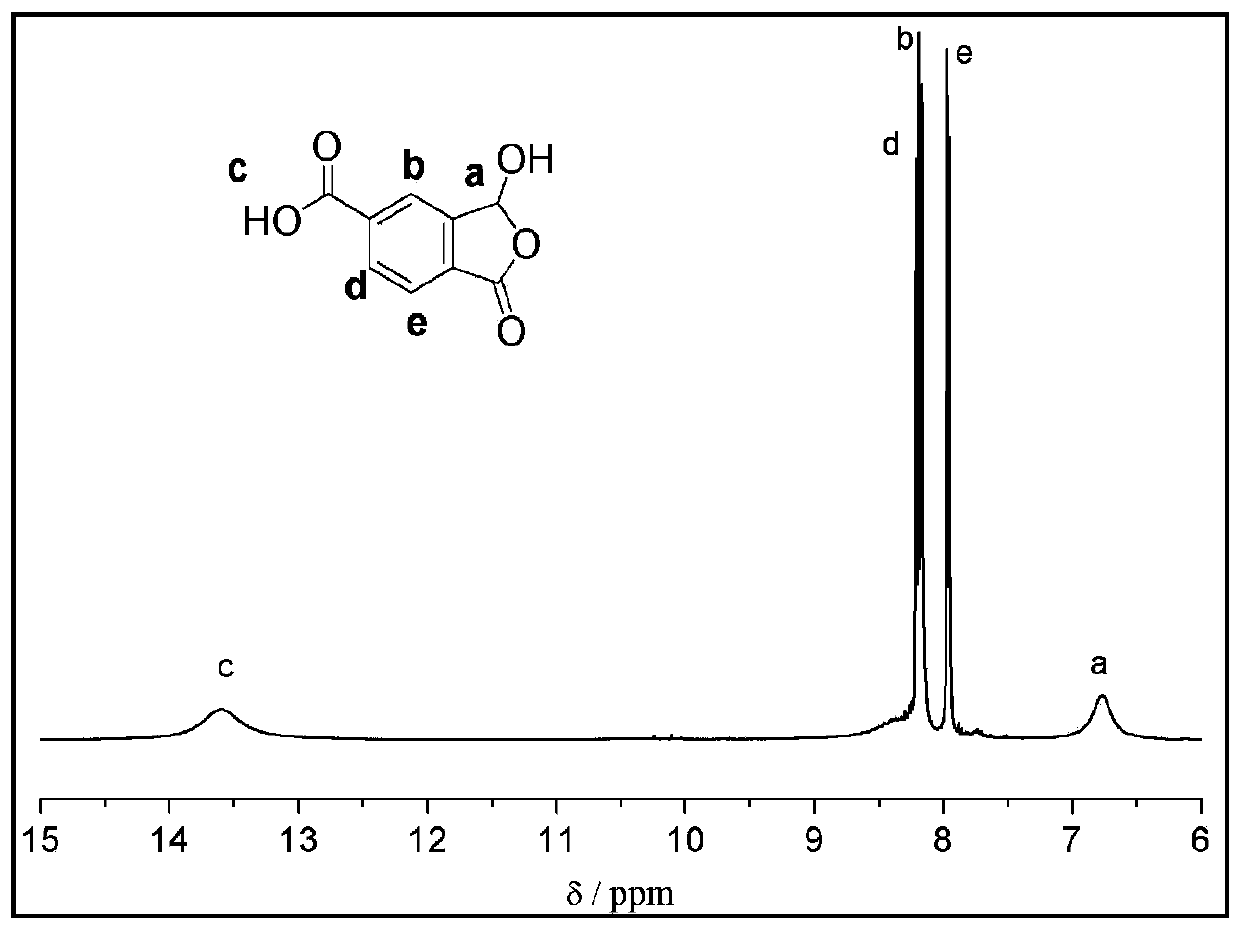
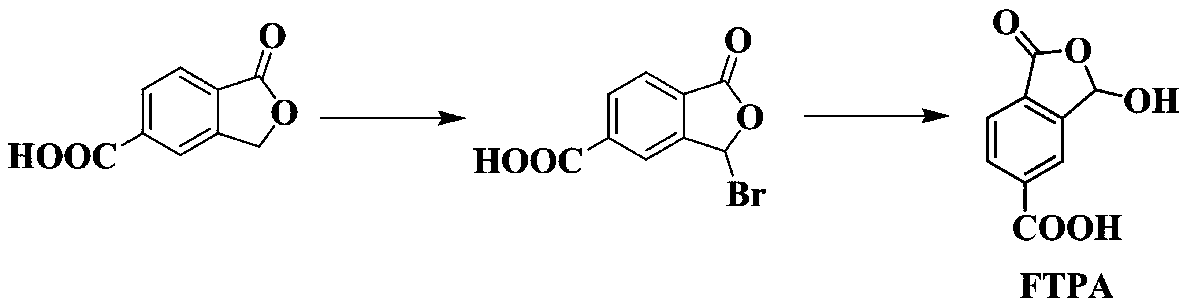
Method for synthesizing 1-oxo-1,3-dihydro-3-hydroxybenzofuran-5-carboxylic acid
A technology of hydroxybenzene and dihydrogen, applied in the field of organic synthesis, can solve the problems of undeveloped methods and related patents, and achieve the effects of short reaction time, easy availability of raw materials, and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation method of 1-oxo-1,3-dihydro-3-hydroxybenzofuran-5-carboxylic acid, the steps are as follows:
[0043](1) Add 2,5-dibromotoluene (14.5g, 0.058mol), glacial acetic acid (87.07g, 1.45mol), acetic anhydride (77.8g, 0.76mol), concentrated sulfuric acid (30.8g, 0.31mol) in sequence In a 250mL single-necked flask, the reaction system was cooled to 0-5°C in an ice-water bath. Under vigorous stirring, chromium trioxide (17.5g, 0.175mol) was slowly added in batches, and the addition process lasted for 30min. The reaction temperature was controlled by an ice-water bath at 0-5°C; the final reaction solution continued to stir at this temperature for 2h, and then Under stirring for 2h. The reaction solution was slowly poured into ice water (850mL), and stirred rapidly, a large amount of light green solid appeared, placed in ultrasonic vibration for 20min, and suction filtered to obtain a light green solid, which was washed in batches with 200mL deionized water, and t...
Embodiment 2
[0049] The preparation method of 1-oxo-1,3-dihydro-3-hydroxybenzofuran-5-carboxylic acid, the steps are as follows:
[0050] (1) Add 2,5-dichlorotoluene (9.34g, 0.058mol), glacial acetic acid (87.08g, 1.45mol), acetic anhydride (88.82g, 0.87mol), concentrated sulfuric acid (46.1g, 0.46mol) in sequence In a 250mL single-necked flask, the reaction system was cooled to 0-5°C in an ice-water bath. Under vigorous stirring, chromium trioxide (29g, 0.29mol) was slowly added in batches, and the addition process continued for 30 minutes. The ice-water bath controlled the reaction temperature at 0-5°C; the final reaction solution continued to stir at this temperature for 2 hours, and then Stir for 24h. The reaction solution was slowly poured into ice water (1700mL), and stirred rapidly, a large amount of light green solid appeared, placed in ultrasonic vibration for 20min, and suction filtered to obtain a light green solid, which was washed in batches with 200mL deionized water, and th...
Embodiment 3
[0055] The preparation method of 1-oxo-1,3-dihydro-3-hydroxybenzofuran-5-carboxylic acid, the steps are as follows:
[0056] (1) The difference from step (1) of Example 1 is that the amount of chromium trioxide is 23.2g, 0.232mol, and the obtained solid is uniformly dispersed with petroleum ether, and the mixed solution is shaken in an ultrasonic instrument for 1h, and suction filtered to obtain white 19.3 g of solid 2,5-dibromo-diacetoxymethylbenzene, yield 91%.
[0057] (2) The difference from Example 1 step (2) is that the palladium reagent is palladium chloride (71mg, 0.4mmol), the phosphine ligand is tetrakistriphenylphosphine (463mg, 0.8mmol), and the base is trimethylamine ( 6mL), the reaction time was 4h, 1.28g of 2-formyl terephthalic acid was obtained, and the yield was 82%.
[0058] (3) The difference from step (3) of Example 1 is that the palladium reagent is [2,2'-bis(diphenylphosphine)-1,1'-binaphthyl]palladium dichloride (320mg, 0.4mmol ), the phosphine ligand...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com