An anti-tumor compound and its preparation method, application, and pharmaceutical compositions
An anti-tumor and compound technology, applied in the field of medicine, can solve problems such as low efficacy, cytotoxicity, and difficulty in reaching the level of cytotoxicity of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
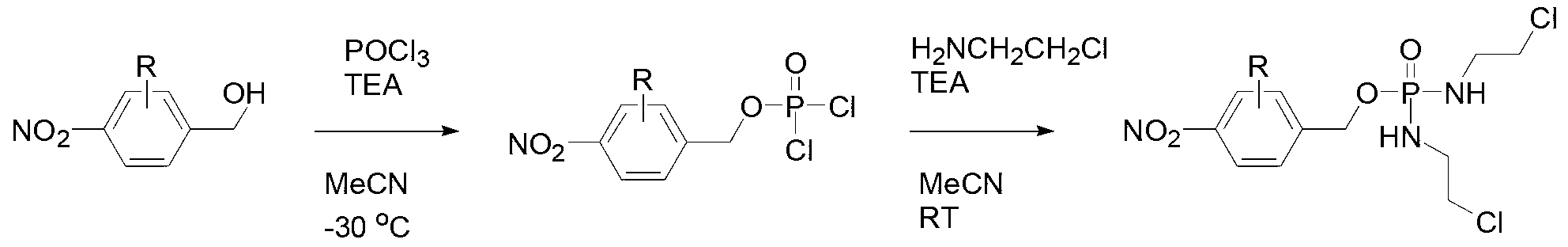
[0034] see image 3 , which is a flowchart of the preparation method of the antitumor compound of the present invention. The preparation method of this antitumor compound comprises the following steps:
[0035] Phosphorus oxychloride (POCL 3 ) was dissolved in acetonitrile (MeCN), cooled to minus 30°C, and reacted with 4-nitro-substituted benzyl alcohol and triethylamine (TEA) to form a reaction mixture with structural formula II, which is:
[0036] ;
[0037]Structural formula is that the reaction mixture of II is dissolved in acetonitrile, adds 2-chloroethylamine hydrochloride (H 2 NCH 2 CH 2 CL) and triethylamine, stirred overnight at room temperature (RT);
[0038] Triethylamine hydrochloride was removed by filtration, and the filtrate was concentrated, separated and purified by silica gel chromatography, suction filtered, and dried to form a solid antitumor compound with structural formula I.
[0039] The antitumor compound of the present invention can be applied...
Embodiment 1
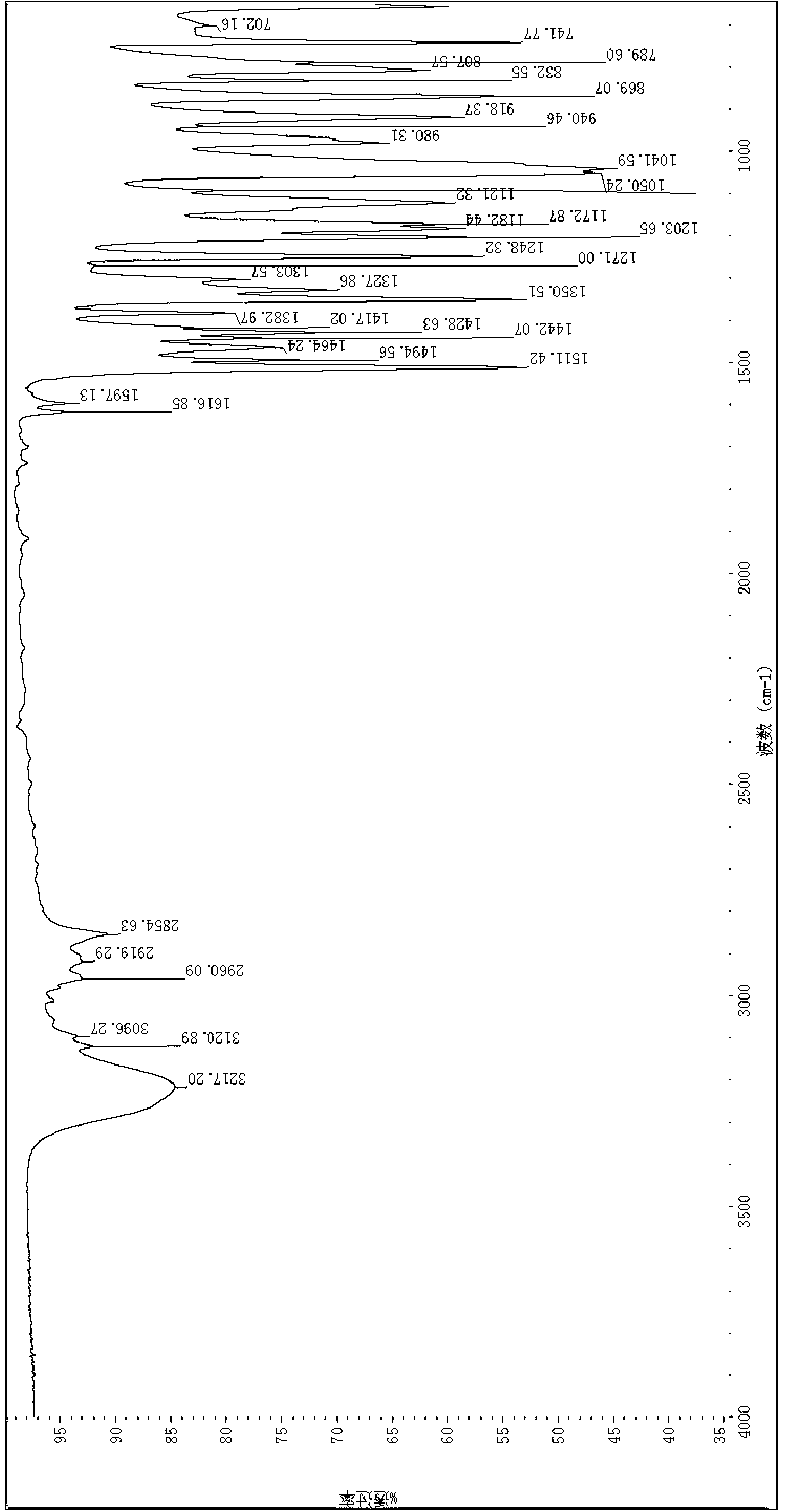
[0046]
[0047] Preparation of 4-nitro-2-chlorobenzyl N,N'-bis(2-chloroethyl)phosphoramidate: Add 10 g (65 mmol) of phosphorus oxychloride to a 1-liter three-necked flask and 40 ml of acetonitrile, cooled to minus 30°C. In a 1-liter three-necked flask, add 13 g of 4-nitro-2-chlorobenzyl alcohol (65 mmol) and 9.1 mL of triethylamine (65 mmol), and 20 mL of acetonitrile as solvent. Into the cooled solution of phosphorus oxychloride, 4-nitro-2-chlorobenzyl alcohol / triethylamine solution, keeping the reaction temperature at -30°C for 15 minutes. Thereafter, to the above reaction mixture, 15.1 g of 2-chloroethylamine hydrochloride (130 mmol) was added, followed by 36.3 ml of triethylamine (262 mmol). The reaction mixture was allowed to warm to room temperature and stirred overnight. The reaction mixture was filtered to remove triethylamine hydrochloride, concentrated by rotary evaporation, and separated and purified by silica gel chromatography. The product is a white solid, ...
Embodiment 2
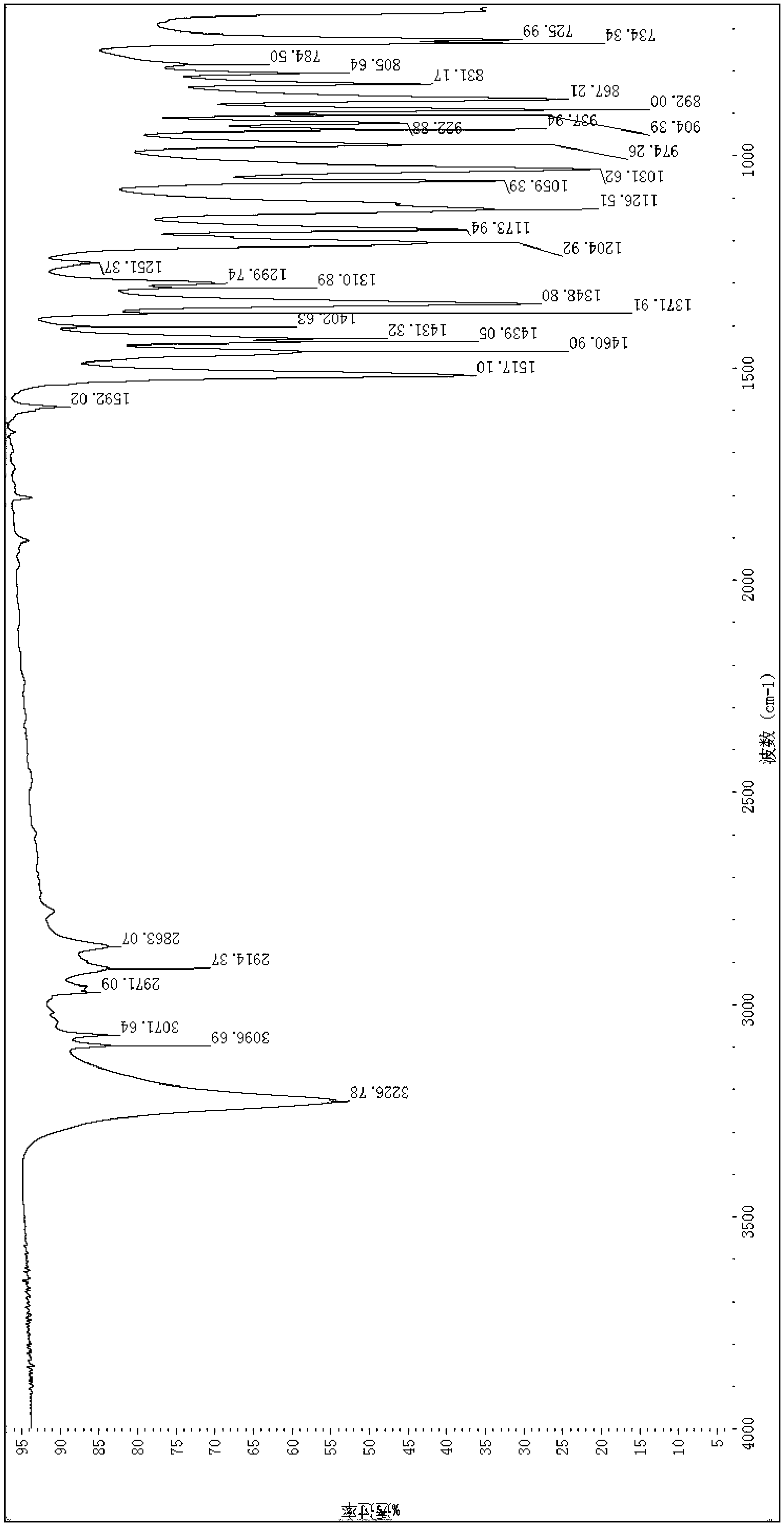
[0051]
[0052] Preparation of 4-nitro-2-methoxybenzyl N,N'-bis(2-chloroethyl)phosphoramidate: Add 10 g (65 mmol) of trichloride to a 1-liter three-necked flask Oxonphosphorus and 40 ml of acetonitrile were cooled to minus 30°C. In a 1-L three-necked flask, add 11.9 g of 4-nitro-2-methoxybenzyl alcohol (65 mmol) and 9.1 mL of triethylamine (65 mmol), and 20 mL of acetonitrile for solvent. Into the cooled solution of phosphorus oxychloride, 4-nitro-2-methoxybenzyl alcohol / triethylamine solution, keeping the reaction temperature at -30°C for 15 minutes. Thereafter, to the above reaction mixture, 15.1 g of 2-chloroethylamine hydrochloride (130 mmol) was added, followed by 36.3 ml of triethylamine (262 mmol). The reaction mixture was allowed to warm to room temperature and stirred overnight. The reaction mixture was filtered to remove triethylamine hydrochloride, concentrated by rotary evaporation, and separated and purified by silica gel chromatography. The product is a wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com