Silkworm recombination baculovirus expressing paraoxonase 1 gene and preparation method and application thereof
A technology of recombinant baculovirus and paraoxonase, applied in microorganism-based methods, botanical equipment and methods, biochemical equipment and methods, etc., can solve problems such as unpurified recombinant proteins, and achieve high expression, high Good security and the effect of solving insufficient sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Synthesis of PON1 gene
[0045] According to the PON1 gene sequence published by GenBank accession number AY499193.1, a pair of primers were designed to amplify the PON1 gene (italics are restriction sites), and its nucleotide sequence is shown in SEQ ID NO:1.
[0046] Upstream primer F: 5'-TAT GAATTC ATGGCTAAACTGACAGCG-3’, where italics are Eco RI site;
[0047] Downstream primer R: 5'-GCG CTCGAG TTACAGCTCACAGTAAAG-3', where italics are xho I site;
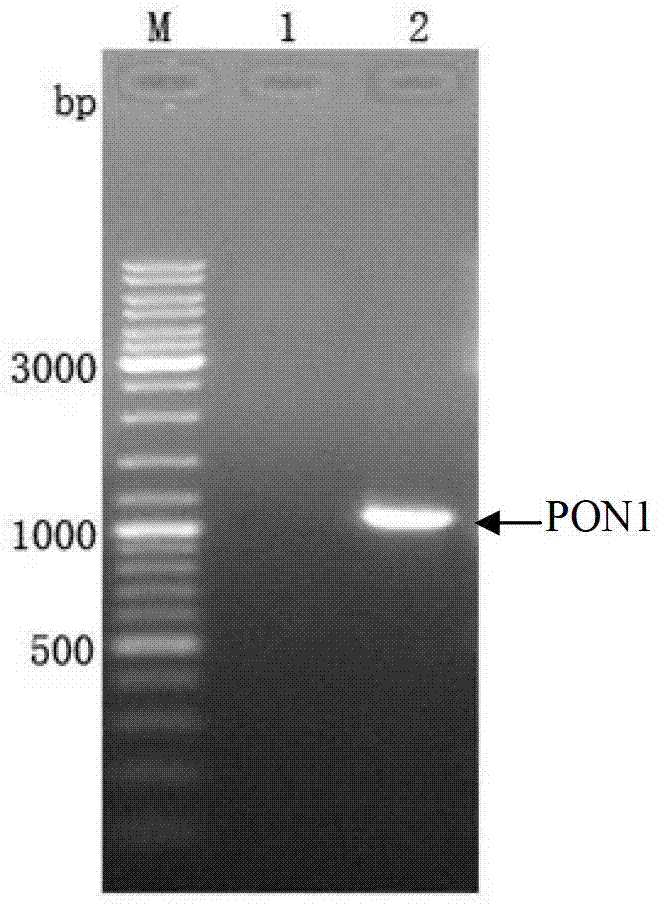
[0048] Using the pBlueScript-PON1 plasmid (Teng Xia, Yang Shen, etc. Expression of human serum Q-type paraoxonase in Escherichia coli. [J] Biotechnology Communications 2007, 18 (1): 015-018) as a template, using the above Primers were used for PCR amplification and agarose gel electrophoresis (such as figure 1 As shown), the size of the gene fragment of PON1 is about 1065bp, and agarose gel electrophoresis shows that the size of the PCR product of the target gene in corridor 2 is consistent with the ac...
Embodiment 2
[0059] Example 2: Recombinant transfer vector pFastBac TM Construction of HTA-PON1
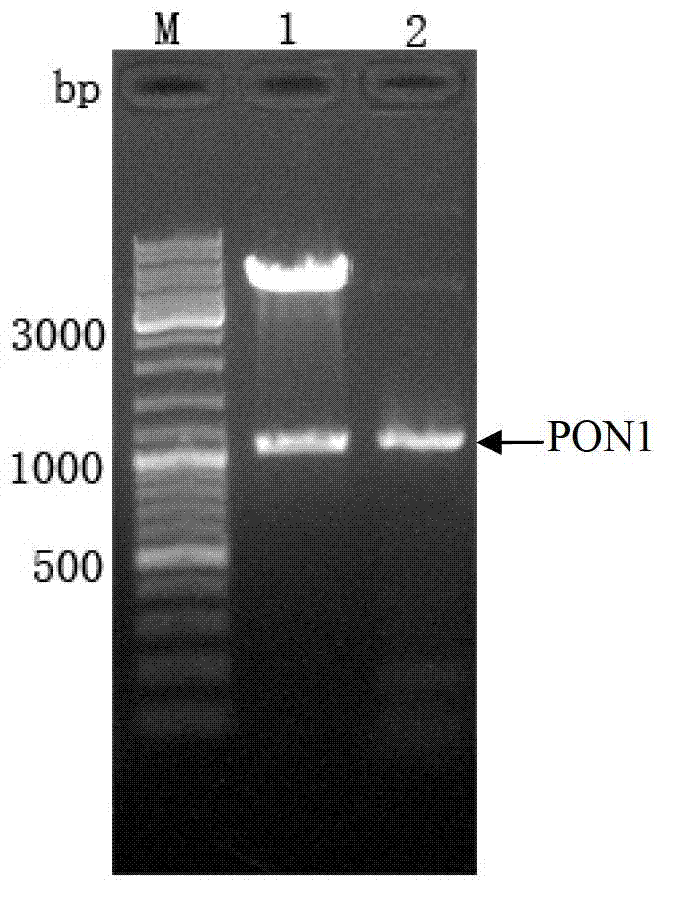
[0060] PON1 gene and pFastBac obtained in embodiment 1 TM For HTA vector (Invitrogen) EcoR I and xho Ⅰ (Fermentas Company) performed double enzyme digestion at the same time, and agarose gel electrophoresis was used to recover the digested product; the recovered PON1 gene fragment was connected to the digested pFastBac with Ligation high ligase (Fermentas Company) TM On the HTA vector, make the target gene (PON1 gene) under the control of the polyhedron promoter, transform Escherichia coli TG1 competent cells (Fermentas company), screen positive clones, and obtain the recombinant transfer vector pFastBac TM HTA-PON1. The recombinant transfer vector was carried out by PCR and double enzyme digestion, and the product was identified by agarose gel electrophoresis (such as figure 2 shown), two bright bands appear in the double enzyme digestion product of No. 1 lane, one of which is the 106...
Embodiment 3
[0061] Embodiment 3: Construction of Bombyx mori recombinant baculovirus BmNPV-PON1
[0062] 1) Obtaining recombinant baculovirus DNA (Bacmid-PON1)
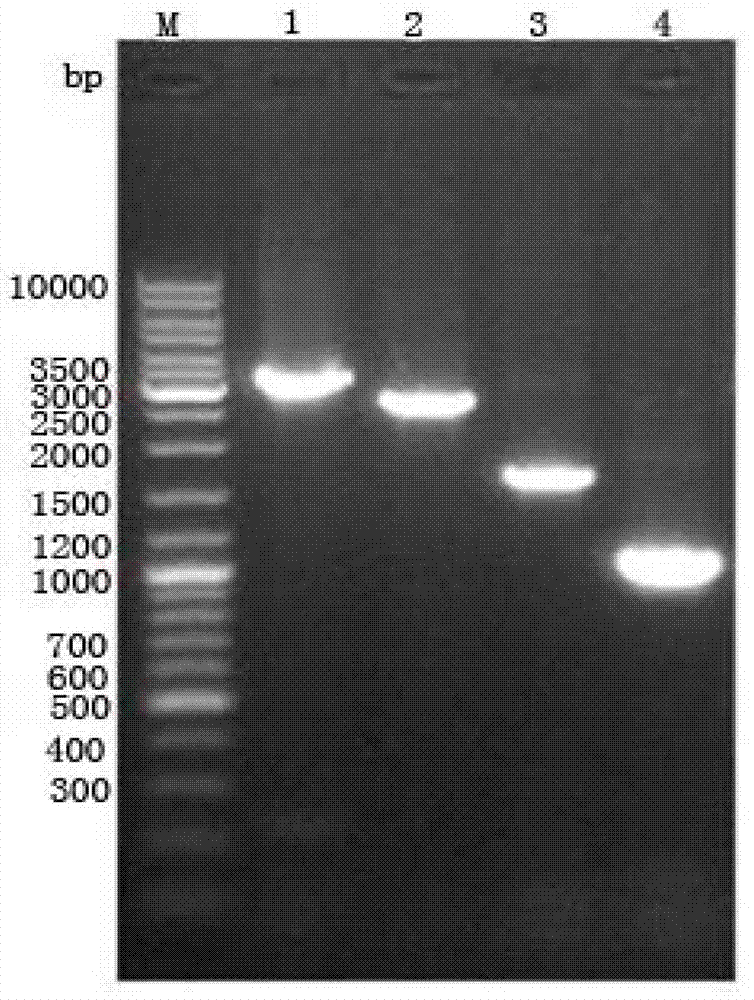
[0063] The recombinant transfer vector pFastBac constructed in Example 2 TM HTA-PON1 was transformed into Escherichia coli DH10Bac competent cells (Fermentas company), and positive clones were obtained by blue-white screening. PCR was performed using the combination of M13 universal primers and the upstream and downstream primers of the target gene, and a small amount of PCR products were taken for agarose gel electrophoresis. (Such as image 3 Shown), electrophoresis results showed that the correct Bacmid-PON1 positive clones were obtained.
[0064] 2) Recombinant baculovirus DNA (Bacmid-PON1) transfected silkworm BmN cells
[0065] a. Take 1ml of silkworm BmN cells in good growth state with a pipette 8-12 hours in advance and spread them in a small cell culture dish (3.5cm in size);
[0066] b. Take 10 μL of recombinant Bac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com