Design of multifunctional self-powered electrochromic material and integrated electrochromic device
A technology of electrochromic materials and electrochromic layers, applied in the direction of color-changing fluorescent materials, electric solid devices, electrical components, etc., can solve the problems of triphenylamine derivatives that have not been reported, and achieve the effect of low preparation cost and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
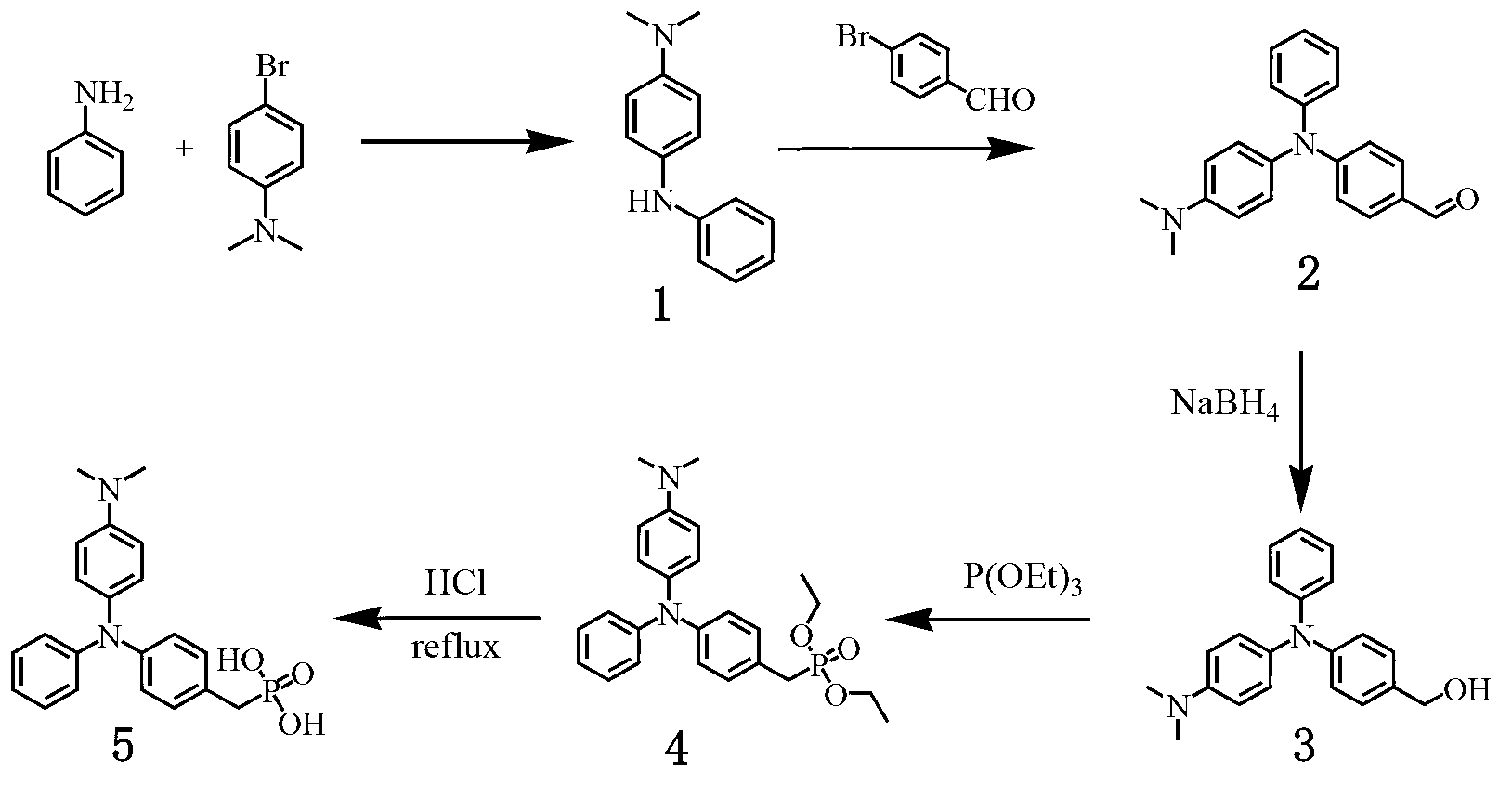
[0039] Example 1 Preparation of triphenylamine self-powered electrochromic material
[0040](1) Synthesis of compound 1: under the protection of inert gas, weigh 2.73ml (30mmol) aniline, 4.066g (20mmol) 4-bromo-N,N-dimethylaniline, 0.12g (0.13mmol) tris(di Benzylideneacetone) dipalladium, 0.0531g (0.26mmol) tri-tert-butylphosphine, and 1.922g (20mmol) sodium tert-butoxide were added to toluene. React at 90°C for 24 hours. Toluene was removed under reduced pressure and dissolved with chloroform. After filtration, it was washed 3 times with saturated brine. The organic layer was dried, filtered, spin-dried, and a yellow product was obtained by column analysis.
[0041] (2) Synthesis of compound 2: under the protection of an inert gas, weigh 3.162g (14.89mmol) of compound 1, 4g (21.62mmol) of p-bromobenzaldehyde, 0.18g (0.20mmol) of tris(dibenzylideneacetone) di Palladium, 0.0795g (0.40mmol) tri-tert-butylphosphine, 4g (20mmol) cesium carbonate were added to toluene. React a...
Embodiment 2 3
[0045] Example 2 Preparation of triphenylamine self-powered electrochromic material
[0046] (1) Synthesis of compound 1: under the protection of an inert gas, weigh 1.90ml (20mmol) aniline, 4.066g (20mmol) 4-bromo-N,N-dimethylaniline, 0.12g (0.13mmol) three (di Benzylideneacetone) dipalladium, 0.053g (0.26mmol) tri-tert-butylphosphine, and 1.92g (20mmol) sodium tert-butoxide were added to toluene. React at 90°C for 24 hours. Toluene was removed under reduced pressure and dissolved with chloroform. After filtration, it was washed 3 times with saturated brine. The organic layer was dried, filtered, spin-dried, and a yellow product was obtained by column analysis.
[0047] (2) Synthesis of compound 2: under the protection of an inert gas, weigh 3.162g (15mmol) of compound 1, 2.78g (15mmol) of p-bromobenzaldehyde, 0.18g (0.20mmol) of tris(dibenzylideneacetone)dipalladium , 0.0795g (0.40mmol) tri-tert-butyl phosphorus, 4g (20mmol) cesium carbonate were added to toluene. React...
Embodiment 3
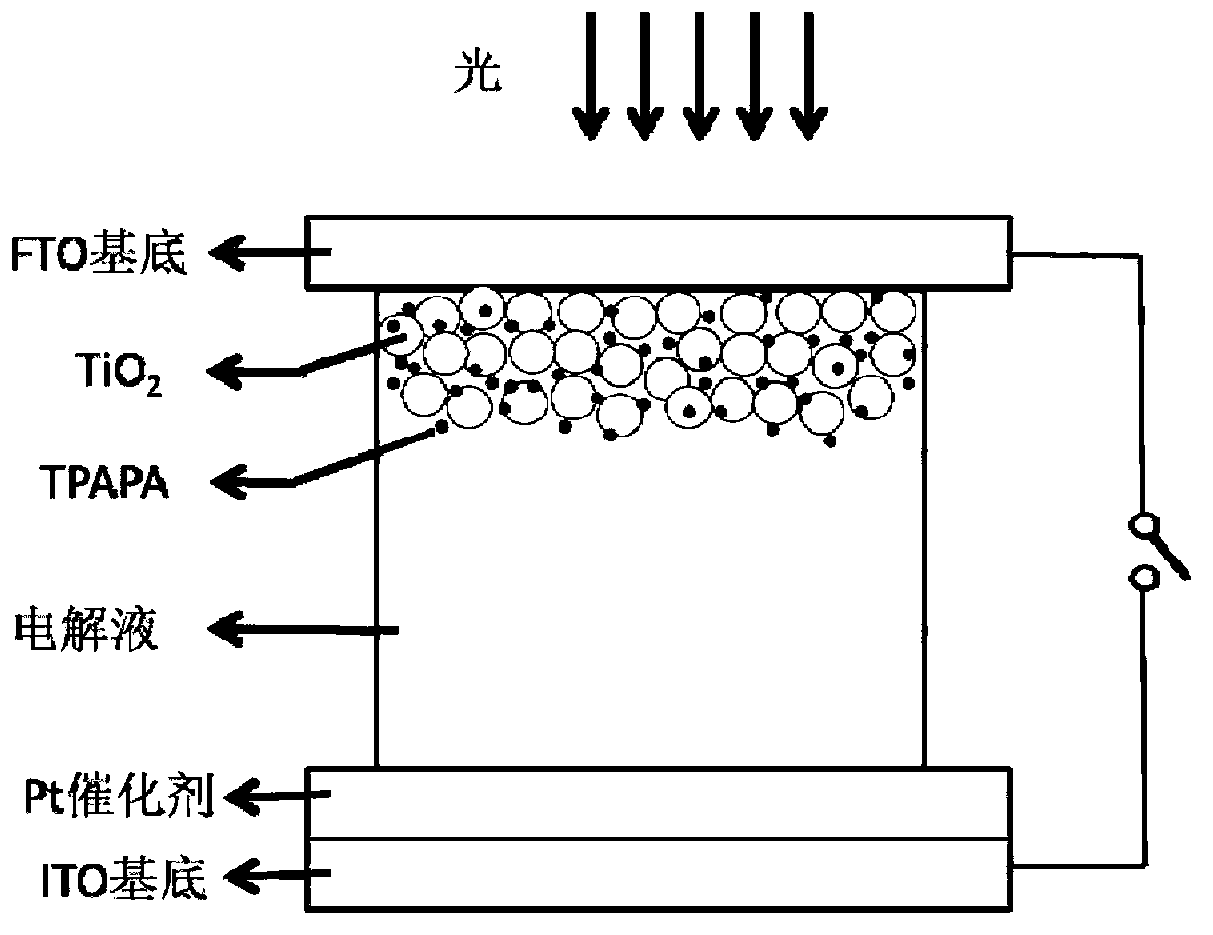
[0051] Embodiment 3 Assembly of self-powered electrochromic device
[0052] (1) Preparation of counter electrode
[0053] Apply the pt catalyst gel to the conductive surface of the transparent conductive substrate ITO, heat it at 260°C for 10 minutes, take it out, and cool it to room temperature to obtain the counter electrode.
[0054] (2) Preparation of working electrode
[0055] Use the scraper method to coat the porous semiconductor film on the conductive surface of the transparent conductive substrate FTO, heat it at 400~450°C for 30 minutes, and place it in the material solution synthesized in Example 1 for adsorption. The solution is preferably chloroform as a solvent, and the adsorption time is preferably 24 to 48 hours to ensure that the adsorption reaches the optimum value, so as to obtain the working electrode.
[0056] (3) Choice of electrolyte
[0057] Through comparative experiments of different electrolytes, the preferred electrolyte is Iodolyte AN-50.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com