Kit for detecting vasculitis related autoantibody repertoire
An autoantibody and antibody detection technology, applied in the biological field, can solve the problems of easy misjudgment and missed judgment, cannot be used as a basis for diagnosis, low specificity, etc., and achieves the effect of uniform width and simple and reliable result judgment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
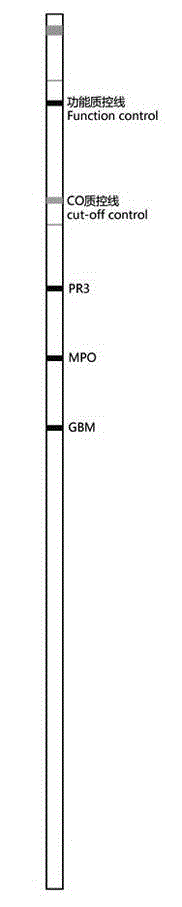
[0035] see Figure 1 to Figure 2 as shown, figure 2 The CO quality control line in the sample is the critical quality control zone; the kit for detecting vasculitis-related autoantibody spectrum in this embodiment includes membrane strips, enzyme labeling solution, substrate and concentrated washing incubation solution, wherein the enzyme labeling solution, substrate The selection and scope of the concentrated washing and incubation solution all belong to the prior art. For the enzyme label solution, the substrate and the concentrated washing incubation solution, the technical scheme of the present invention can also be realized by selecting the products of the prior art.
[0036] The membrane strip is composed of slides and antigen strips, critical quality control strips, and functional quality control lines that are sequentially fixed on the slides. The antigen strips are drawn from at least two independent lines of PR3, MPO and GMB to nitrocellulose Formed on a membrane o...
Embodiment 2
[0044] This example is an experimental determination of the antigen bands formed by three independent streaks of PR3, MPO and GMB on nitrocellulose or nylon membranes. On the basis of Example 1, in order to make the result judgment more simple and reliable, the width of each antigen strip is uniformly set, and the interval between the strips is equal. The width of the antigen strip is 0.5mm-3mm, and the interval between adjacent antigen strips 2mm-20mm, the background of the membrane strip is clean, and an improved single-reagent substrate is used, which does not need to be terminated after adding the substrate. Reagents; all imprinted antigens are highly purified, and the latest international coating technology is used. The specific coating method is described later.
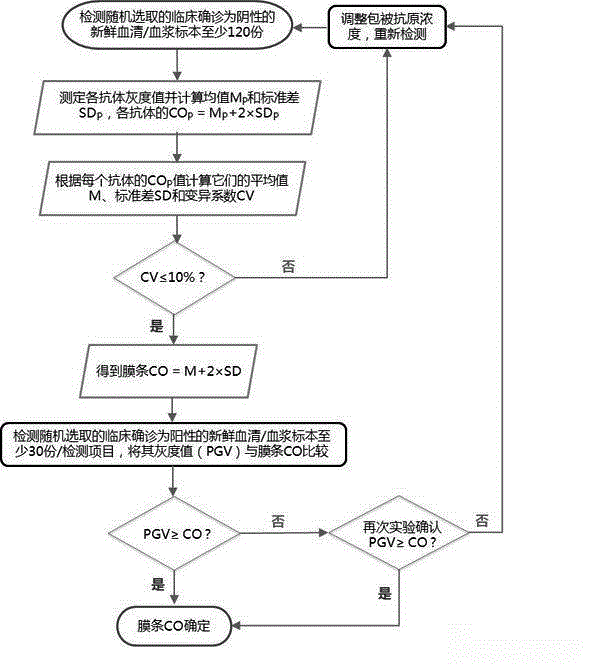
[0045] Determination of critical quality control values:
[0046] 1) Determine the critical quality control value of each antibody
[0047] Randomly select 123 fresh serum and plasma samples clinically diagno...
Embodiment 3
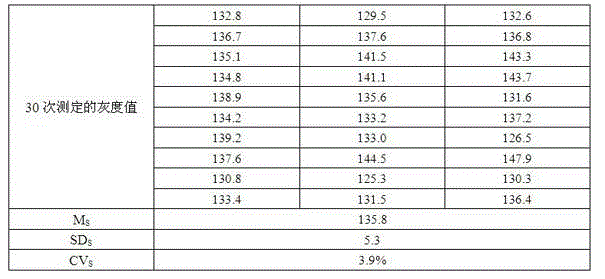
[0056] This embodiment is the critical quality control value of the membrane strip determined according to embodiment 2 (in figure 2 and in all examples of the present invention, CO p is the critical quality control value of a single antibody, CO is the critical quality control value of the entire membrane strip), to adjust the concentration of human IgG coated in the critical quality control zone, and finally determine the coating concentration and coating process of the critical quality control zone. The specific operation is: dissolve a certain concentration of human IgG in Tris or Hepes buffer, then use a fully automatic spotting instrument to streak on the nitrocellulose membrane, and prepare finished membrane strips through sealing, drying, cutting and other processes. And only the critical quality control band is coated on the film strip. Randomly select 30 membrane strips to test the random negative samples of step 1), and scan the gray scale, and calculate the mean ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com