Nanoscale capecitabine and preparation method thereof
A capecitabine nano-scale technology, applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., can solve problems such as hair loss, liver function damage, inhibition, etc., achieve structural stability, production The effect of simple method and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
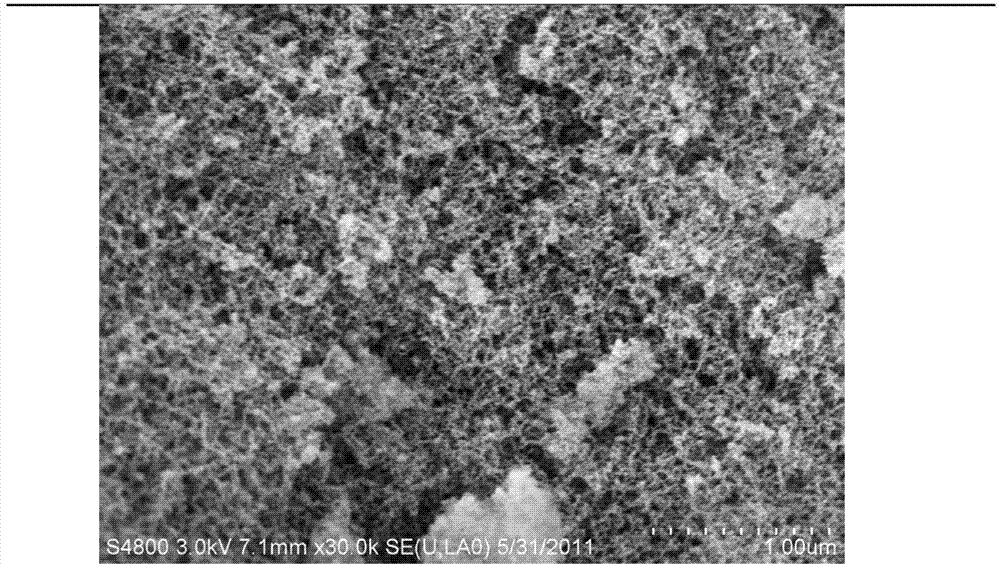
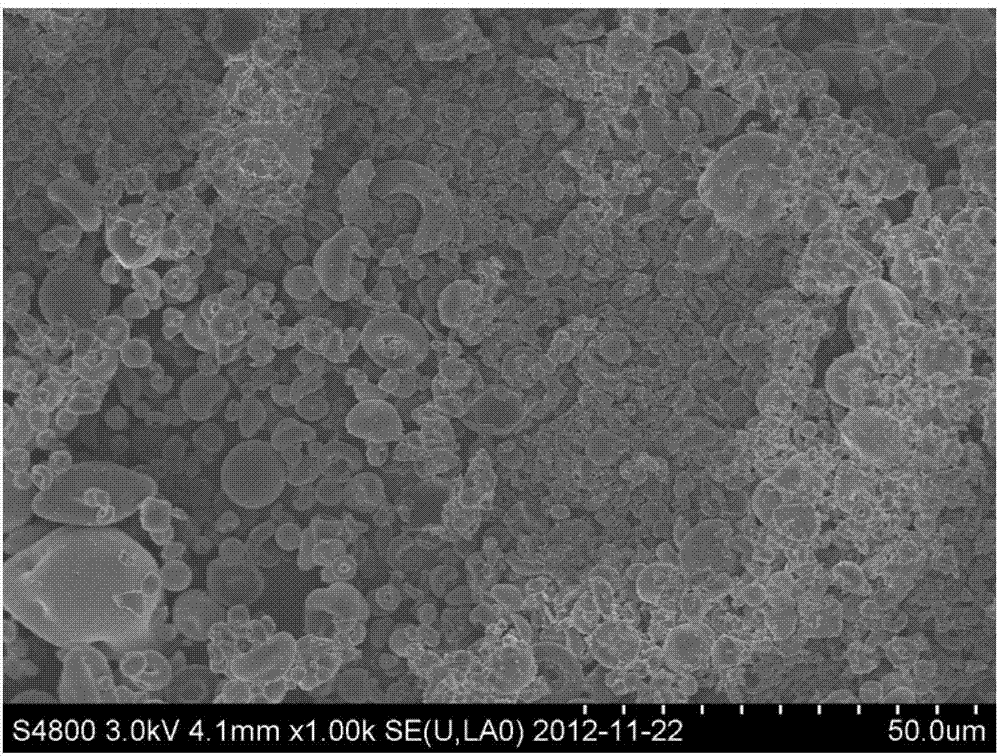
Image
Examples
Embodiment 1
[0074] The nanoscale capecitabine of the present embodiment is prepared according to the following method:
[0075] 1. Capecitabine API (Jinan Fuchuang Pharmaceutical Technology Co., Ltd.) 1g, add 20ml of absolute ethanol to dissolve;
[0076] 2. Add 2g of silica airgel after heat treatment at 500°C for adsorption;
[0077] 3. After the adsorption is complete, dry it in an oven at 60°C;
[0078] 4. After drying, add 100ml of pure water and emulsify with 25000rpm / min ordinary emulsifier for 5min;
[0079] 5. High-pressure homogenizer (Shanghai Donghua GYB 30-6S), 400bar, cycle 6 times, 10min;
[0080] 6. Spray-dry the homogeneous liquid in an experimental spray dryer (Shanghai Shunyi Technology SP-1500), parameters: temperature 130°C, flow rate 500ml / h, nozzle: 0.75mm, and obtain nanoscale capecitabine particles after drying .
Embodiment 2
[0082] The nanoscale capecitabine of the present embodiment is prepared according to the following method:
[0083] 1. Capecitabine API (Jinan Fuchuang Pharmaceutical Technology Co., Ltd.) 1g, add 5ml of absolute ethanol to dissolve;
[0084] 2. Add 0.5g of silica airgel after heat treatment at 1000℃ for adsorption;
[0085] 3. After the adsorption is complete, dry naturally;
[0086] 4. After drying, add 20ml of pure water and emulsify with an ordinary emulsifier at 25000rpm / min for 5min;
[0087] 5. High pressure homogenizer (Shanghai Donghua GYB 30-6S), 400bar, cycle 8 times, 10min;
[0088] 6. Spray-dry the homogeneous liquid in an experimental spray dryer (Shanghai Shunyi Technology SP-1500), parameters: temperature 130°C, flow rate 500ml / h, nozzle: 0.75mm, and obtain nanoscale capecitabine particles after drying .
Embodiment 3
[0090] The nanoscale capecitabine of the present embodiment is prepared according to the following method:
[0091] 1. Capecitabine API (Jinan Fuchuang Pharmaceutical Technology Co., Ltd.) 1g, add 150ml of absolute ethanol to dissolve;
[0092] 2. Add 15g of hydrophilic silica airgel for adsorption;
[0093] 3. After the adsorption is complete, freeze-dry;
[0094] 4. After drying, add 150ml of pure water and emulsify with 25000rpm / min ordinary emulsifier for 5min;
[0095] 5. High-pressure homogenizer (Shanghai Donghua GYB 30-6S), 400bar, cycle 7 times, 10min;
[0096] 6. Spray-dry the homogeneous liquid in an experimental spray dryer (Shanghai Shunyi Technology SP-1500), parameters: temperature 130°C, flow rate 500ml / h, nozzle: 0.75mm, and obtain nanoscale capecitabine particles after drying .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com