Industrialized glycyl-L-glutamine synthetizing method suitable
A technology of glutamine and synthesis method, which is applied in the field of synthesis technology of glycyl-L-glutamine, can solve the problems of unsuitability for industrial production, many reaction steps, and high cost of raw materials, so as to avoid organic residues and simplify the process , the effect of reducing pollution emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
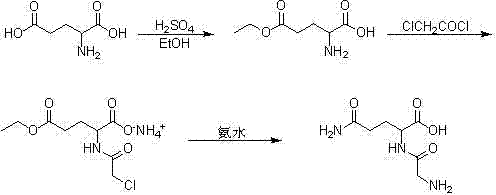
[0007] Mix 5kg of L-glutamic acid with 50L of absolute ethanol, stir, cool to 10℃, drop 2.5L of concentrated sulfuric acid, control the temperature to be less than 25℃, stir at 25℃ for 3h after dripping, and drop the temperature to 10℃. 12.5L triethylamine, a large amount of white solid precipitated, filtered, washed the filter cake with absolute ethanol, the solid was recrystallized with 40L absolute ethanol and 10L water, the precipitated solid was washed once with absolute ethanol, and dried at about 40℃. 4.2 kg (99.2%) of pure L-glutamic acid-γ-ethyl ester was obtained, and the yield was 84%.
Embodiment 2
[0009] Dissolve 4kg of L-glutamic acid-γ-ethyl ester in 40L of saturated sodium bicarbonate solution, cool to 0℃, slowly add 2.4L of chloroacetyl chloride dropwise, while adding dropwise saturated sodium bicarbonate solution to maintain the pH The value is around 8. After dropping, continue stirring at 0°C for 30 minutes, wash with 15L ethyl acetate once, separate the ethyl acetate layer, adjust the pH value of the aqueous layer to 1 with concentrated sulfuric acid, and extract 3 times with 20L ethyl acetate Combine the organic layers, wash the organic layer with 20L saturated sodium chloride solution once, dry with anhydrous sodium sulfate for 3h, evaporate the ethyl acetate under reduced pressure at 40°C to obtain an oily substance, dissolve it with 30L absolute ethanol, and add 6L 28 % Concentrated ammonia water, a white solid precipitated, filtered, the solid was washed once with absolute ethanol, dried at about 40 ℃ to obtain pure chloroacetyl-L-glutamic acid-γ-ethyl amine ...
Embodiment 3
[0011] Mix 3kg of chloroacetyl-L-glutamic acid-γ-ethyl amine salt with 30L 28% concentrated ammonia water, heat to 40°C and keep stirring for 2 days, remove excess ammonia under reduced pressure at 40°C, and then lower the temperature to 0 After ℃, drop 65L of absolute ethanol to precipitate a white solid, filter, and recrystallize the solid with 24L of absolute ethanol and 6L of water to obtain 1.65kg (99.4%) of pure glycyl-L-glutamine, the yield is 55%.
[0012] It is proved through experiments that the synthesis process of glycyl-L-glutamine provided by the present invention increases the operability of the process, at the same time increases the reaction yield and synthesis process product refining rate, and effectively reduces the pollution of industrial production after treatment Discharge, avoid detecting the methanol content in organic residues, and overcome the major drawbacks of the existing synthesis process.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com