Porcine parvovirus, vaccine composition and application thereof
A technology of vaccine composition and parvovirus, applied in the direction of antiviral agent, virus/bacteriophage, virus antigen components, etc., can solve the problems of safety, immune efficacy and insufficient immunity period, and achieve long immunity period, good safety, The effect of reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Isolation, cultivation and characteristic determination of porcine parvovirus
[0025] 1. Isolation of porcine parvovirus
[0026] Spleen, lung and other tissue disease materials (positive for porcine parvovirus detected by PCR) were aseptically collected from a pig farm in Nanjing, which was positive for porcine parvovirus. 1000 IU / ml, freeze-thaw 3 times, centrifuge at 12000 r / min for 5 minutes, take the supernatant, and inoculate the passaged ST cells (purchased from ATCC), the inoculation volume is 0.5ml supernatant / 25cm 3 Square flasks were cultured at 37°C for 5 days and then subcultured, during which the cytopathic changes were observed. No lesions appeared in the first generation, and after the culture was frozen and thawed three times, it was transferred to the second generation and cultured, and no lesions appeared. According to the above subculture method, it was found that the third generation of cells had cytopathic changes after 48 hours of cul...
Embodiment 2
[0058] Example 2 Preparation of Porcine Parvovirus Inactivated Vaccine
[0059] 1. ST cell seed propagation and expansion culture: Take out the frozen ST cell tube from the liquid nitrogen tank, put it in a 37°C water bath to thaw quickly, transfer the cells into a centrifuge tube filled with 10ml DMEM solution, and centrifuge at 1000rpm for 5 minutes. Discard the supernatant, suspend the cells with the growth medium, then add to the cell culture flask, at 37°C, 5% CO 2 cultivated under conditions. When the cell coverage reached 100%, the cells were digested with 0.1% trypsin-EDTA solution. Then subculture at a volume ratio of 1:3. The growth medium is DMEM solution containing 10% FCS (newborn calf serum).
[0060] 2. Virus culture: Remove the growth medium from the ST cell culture with a cell growth coverage rate of 35% to 45%. Inoculate the PPV-JS strain virus solution according to the multiplicity of infection of 0.5%, add DMEM solution containing 2% FCS, and incubate ...
Embodiment 3
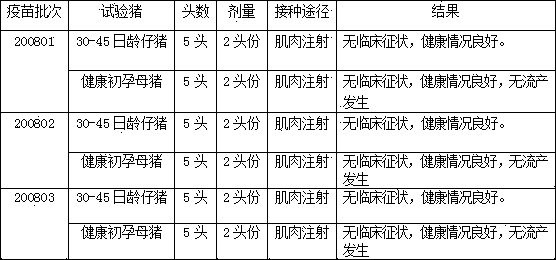
[0070] Example 3 Efficacy Test of Porcine Parvovirus Inactivated Vaccine
[0071] 1. Efficacy test in guinea pigs
[0072] The inoculation objects of each batch of vaccine were grouped as follows: 6 porcine parvovirus HI antibody-negative guinea pigs weighing more than 350 g were taken, 4 of which were used as the immunization group, and 0.5 ml of vaccine was injected intramuscularly into each guinea pig; the other 2 were used as the control group and were not vaccinated.
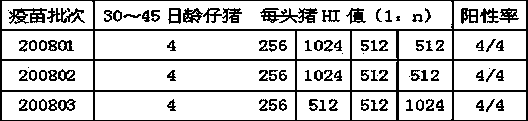
[0073] Twenty-eight days after immunization, blood was collected from each guinea pig to detect porcine parvovirus HI antibody.
[0074] The detection method of porcine parvovirus HI antibody is as follows:
[0075] Antigen working solution preparation: According to the determined antigen HA titer, dilute porcine parvovirus PPV-JS strain culture solution into 4 HA unit antigen with PBS buffer solution to obtain the antigen working solution.
[0076] Treatment of the serum to be tested: Ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com