Method for synthesizing 4A zeolite from kaolin
A technology of kaolin and zeolite, which is applied in the field of synthesizing 4A zeolite from kaolin, can solve the problem of high energy consumption and achieve the effects of high energy consumption, good product quality and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
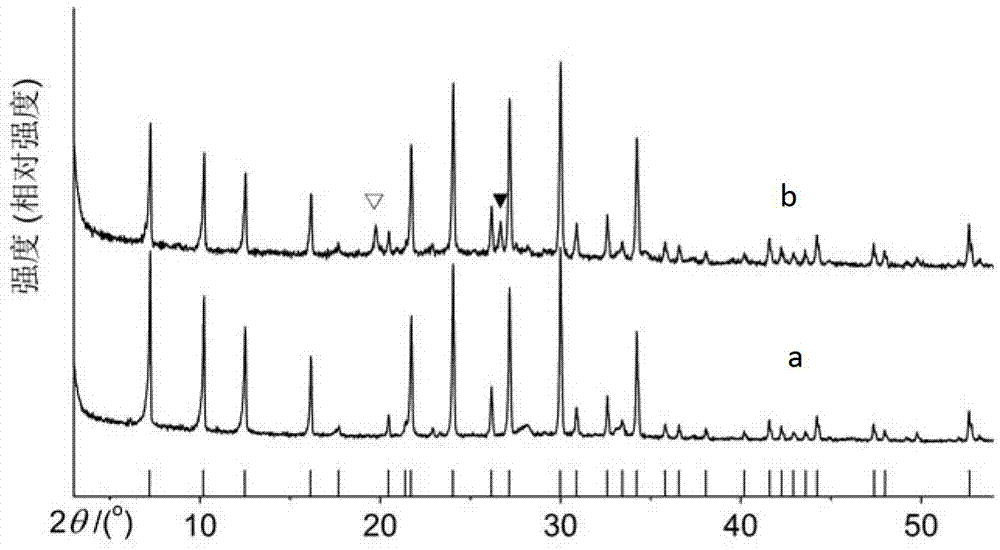
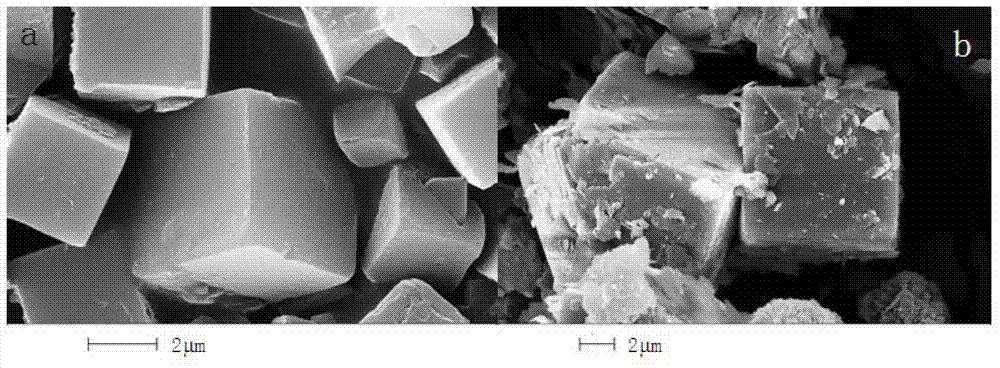
[0022] Select 3g of kaolin, mix it with 16ml of 4mol / L sodium hydroxide solution and put it into the autoclave. The autoclave was kept at 240°C for 3h and then taken out. Then the activated kaolin was dissolved in 40ml of 3mol / L hydrochloric acid, stirred at 50°C for 0.5h and filtered to obtain the kaolin acid hydrolysate. Use NaOH to adjust the pH of the kaolin acid hydrolysis solution to 8 to obtain the active silicon-aluminum raw material, which is mixed with 40 mL of NaOH solution with a concentration of 0.67 mol / L and put into the autoclave. After the autoclave was kept at 90°C for 48 hours, it was washed and filtered to obtain 4A zeolite. Using the national light industry standard (QB / T1768-2003), the calcium ion exchange capacity is 334mg CaCO. 3 / g.
Embodiment 2
[0024] Choose 3g of kaolin, mix it with 16ml of 5mol / L sodium hydroxide solution and put it into the autoclave. The autoclave was kept at 160°C for 24 hours and then taken out. Then the activated kaolin was dissolved in 30ml of 4.8mol / L hydrochloric acid, stirred at 60°C for 1 hour, and filtered to obtain the kaolin acid hydrolysate. Using NaOH to adjust the pH of the kaolin acid hydrolysate to 9 to obtain the active silicon-aluminum raw material, which is mixed with 40 mL of NaOH solution with a concentration of 0.67 mol / L and placed in the autoclave. After the autoclave was kept at 90°C for 48 hours, it was washed and filtered to obtain 4A zeolite. Adopting the national light industry industry standard (QB / T1768-2003) method to measure its calcium ion exchange capacity is 320mg CaCO 3 / g.
Embodiment 3
[0026] Select 3g of kaolin, mix it with 16ml of 4mol / L sodium hydroxide solution and put it into the autoclave. The autoclave was kept at 190°C for 6h and then taken out. The activated kaolin was then dissolved in 100 ml of 1.2 mol / L hydrochloric acid, stirred at 70° C. for 2 hours, and filtered to obtain an acid hydrolysate of kaolin. Adjust the pH of the acid hydrolysis solution to 6 with NaOH to obtain a white flocculent precipitate. The precipitate was mixed with 1mol / L NaOH solution and put into the autoclave. After keeping the autoclave at 90°C for 96h, washing and filtering to obtain 4A zeolite. Adopting the national light industry standard (QB / T1768-2003) method, the calcium ion exchange capacity is 385mgCaCO 3 / g.
[0027] Comparative process: using the same kaolin as the above process, first calcining at 800°C for 4 hours in the traditional process, and using the same synthesis conditions as in Example 3. After the product is kept at 90°C for 96 hours, it is washed a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com