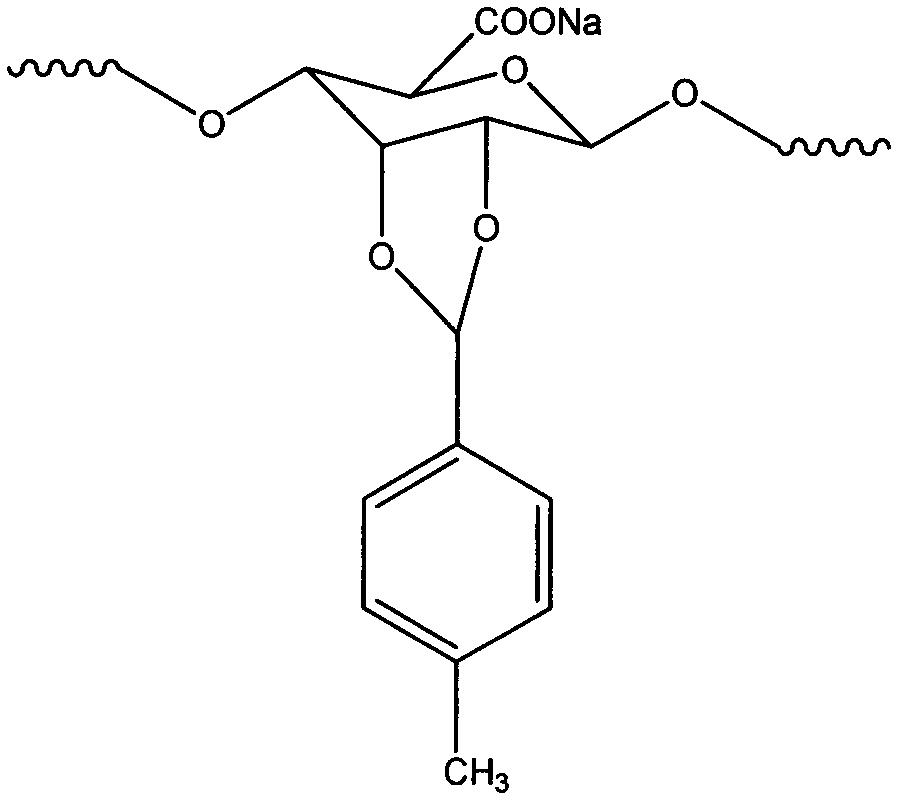
Preparation method for anisic aldehyde-modified sodium alginate and gel microspheres thereof
A technology of sodium alginate and anisaldehyde, applied in medical preparations with non-active ingredients, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve problems such as immeasurable application prospects, and achieve easy control and synthesis process Simple, the effect of increasing the load factor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0020] In a 100ml three-necked flask, prepare 50ml of 2% w / v sodium alginate solution, add 4ml of anisaldehyde to dissolve in 40ml of absolute ethanol, and then pour the solution into the three-necked flask. After sealing, stir electrically until the solution is evenly mixed, then add 2% hydrochloric acid of the monomer mass to catalyze, and carry out two groups of reactions at T=40°C (Scheme I) and 60°C (Scheme II), and the reaction time is 10h. After the reaction, it was precipitated with methanol, finally dissolved in distilled water, dialyzed for 3 days, and freeze-dried to obtain hydrophobically modified sodium alginate.
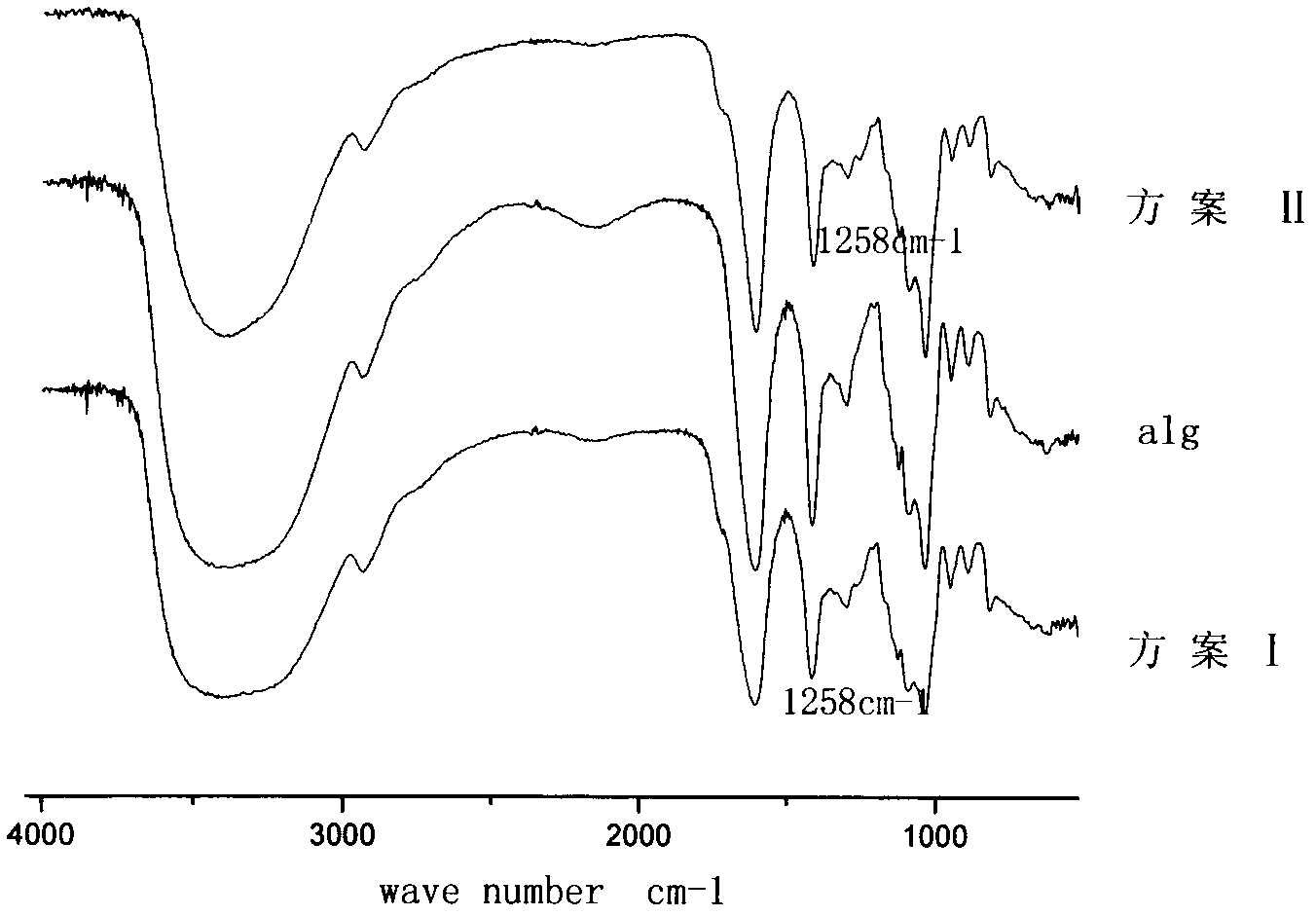
[0021] The occurrence of sodium alginate modification reaction was proved by infrared spectrum. In the infrared spectrum ( figure 2 ), 1258cm -1 is the absorption peak of the C-O ether bond, which indicates that the acetalization reaction between sodium alginate and anisaldehyde occurred.
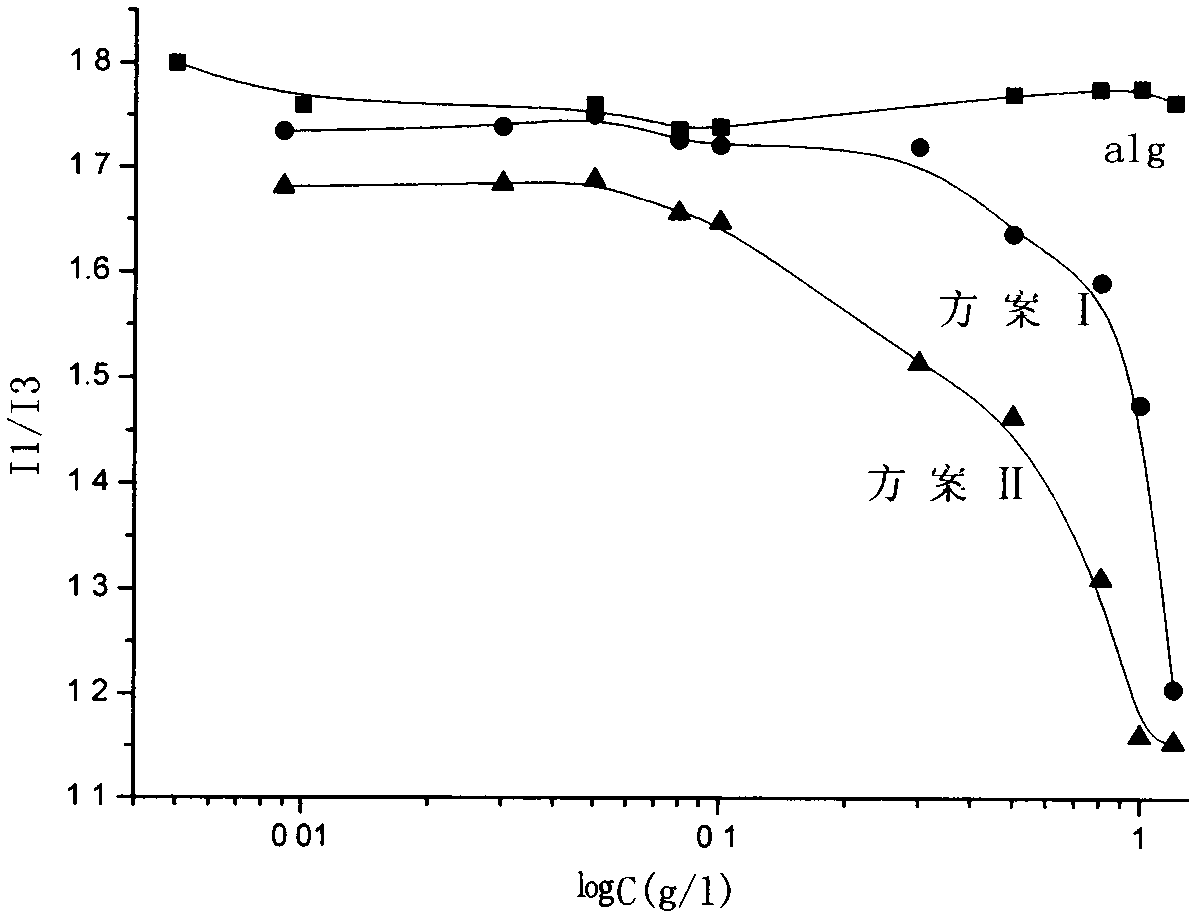
[0022] Fluorescence spectrum ( image 3 ) in I 1 and I 3...
Embodiment approach 2
[0024] Weigh 0.2g of the samples of sodium alginate (alg), scheme I, and scheme II respectively, dissolve it in 10ml of distilled water to obtain a 2% w / v solution, and adjust the pH value of the configured solution to a weak Acidity (in order to compare with sodium alginate), then add 0.02g of the drug bovine serum albumin to be loaded respectively. After fully mixing, under slow stirring with a magnetic stirrer, the solution was slowly dripped into 50ml of CaCl with a mass fraction of 2% using a medical syringe (needle inner diameter 0.45mm). 2 In the solution, continue to solidify for half an hour after the dropwise addition, filter with Buchner funnel, rinse with deionized water three times, and dry the obtained sample at low temperature. Then the in vitro simulated release test was carried out. The gel microspheres prepared by scheme I and scheme II have an embedding rate of 68.3% and 77.93% respectively for the drug bovine serum albumin, while the embedding rate of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com