A kind of Ⅲ crystal form pramipexole hydrochloride tablet and preparation method thereof
A technology for pramipexole hydrochloride and pramipexole hydrochloride tablets, which is applied in the field of III crystal pramipexole hydrochloride tablets and its preparation, and can solve the problems of increasing the load of tableting equipment, poor material fluidity, affecting tablet weight differences, tablet hardness, etc. , to achieve the effect of ensuring stability and avoiding the degradation of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1. According to prescription 1, pass mannitol, corn starch, magnesium stearate, and silicon dioxide through a 100-mesh sieve; and pass through a 200-mesh sieve for pramipexole hydrochloride monohydrate in the third form.
[0042] 2. According to the designed prescription, take 50,000 pieces of auxiliary materials that have passed through a 100-mesh sieve. Preliminarily mixed the weighed III crystal form pramipexole hydrochloride monohydrate raw materials, corn starch, and D-mannitol by the method of doubling increments, then put them in a high-efficiency mixer, mixed for 45 minutes, and tested the content uniformity, qualified Then proceed to the next step.
[0043] 3. After the mixing is completed, put the mixed material into a dry extrusion granulator for dry granulation. (Pre-pressing wheel: 15-20Hz, pressing material: 30-50Hz, feeding material: 10-15Hz, hydraulic pressure: 6-7Mpa). During the granulation process, add material in the hopper at any time. Adjust the...
Embodiment 2
[0048] According to the ratio of prescription 2-8 to make tablets, refer to Example 1 for specific operations.
Embodiment 3
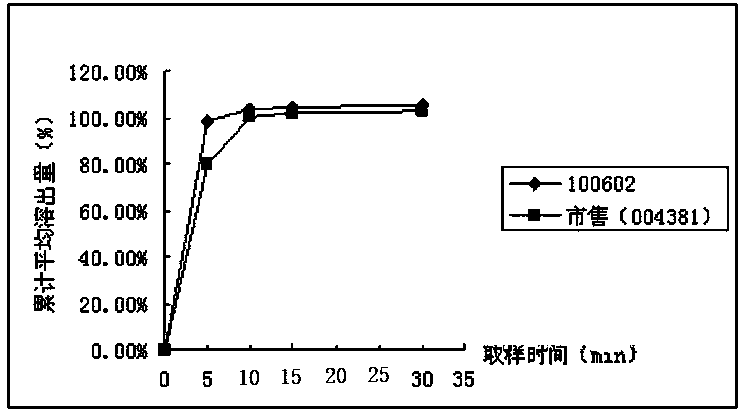
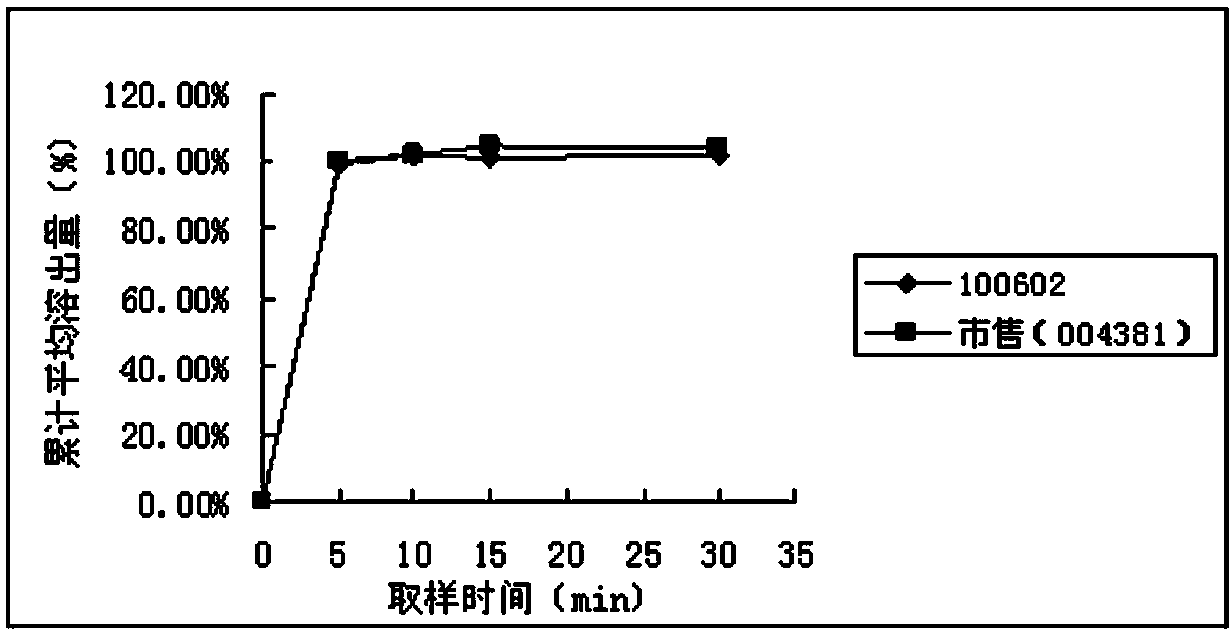
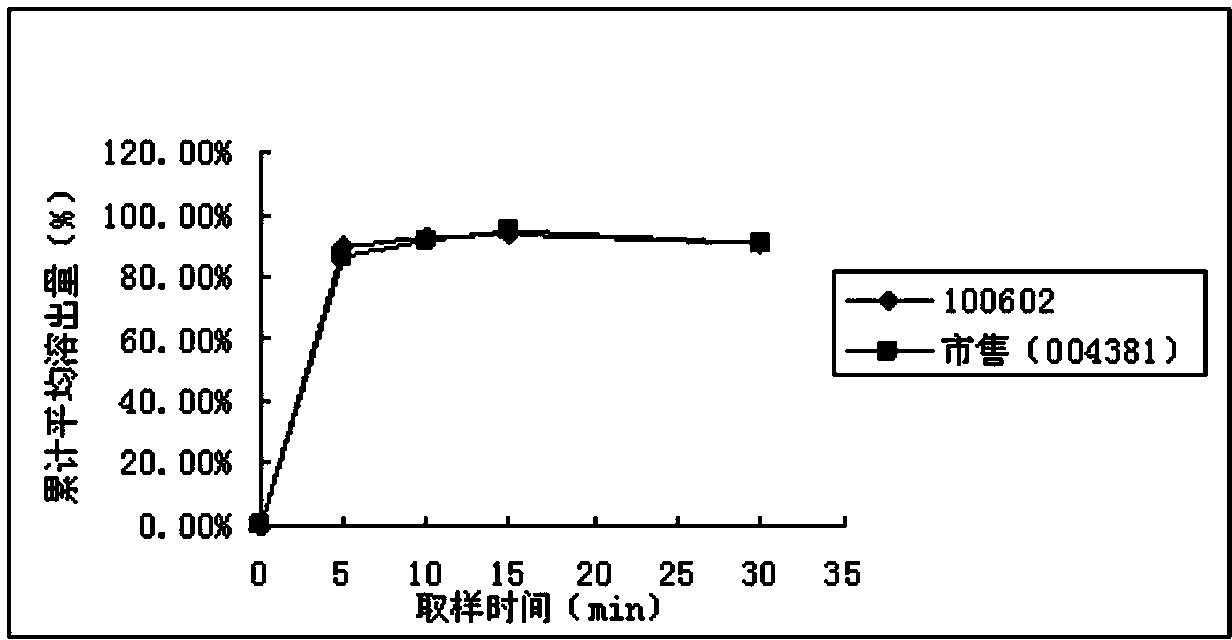
[0050] Pramipexole hydrochloride is easily soluble in water. In the import quality standard (JX20070241), 500ml of citrate / phosphate buffer (PH6.8) is used as the solvent. The paddle method is used at a speed of 50 rpm. This method is consistent with the FDA dissolution database The conditions of the published dissolution method for pramipexole hydrochloride tablets are consistent. The present invention adopts four kinds of solvents (see Table 1) conditions to investigate the similarity (50≤f 2 ≤100) meet the requirements.
[0051] Table 1 Four kinds of solvent conditions
[0052]
[0053] Pramipexole hydrochloride tablets (batch number: 100602, specification: 0.25mg; batch number: 100601, specification: 1.0mg) and pramipexole hydrochloride tablets commercially available in the examples (Senfulol; batch number: 002448; specification: 1.0mg ; Batch number: 004381; Specification: 0.25mg) Carry out dissolution curve research in four kinds of dissolution media and draw dissol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com