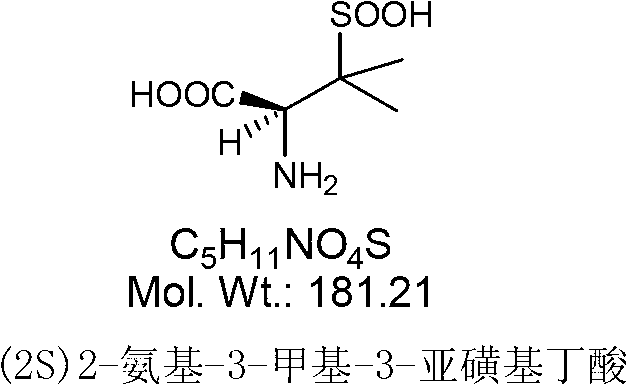
Preparation and crystallization method of (2S)-2-animo-3-methyl-3-sulfinobutanoic acid
A technology of sulfinylbutyric acid and methyl group, which is applied in the field of preparation and crystallization of 2-amino-3-methyl-3-sulfinylbutyric acid, can solve the problem that the product is not easy to separate, unsuitable for long-term storage, complicated to operate, etc. problems, to achieve the effect of high product yield, easy storage, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
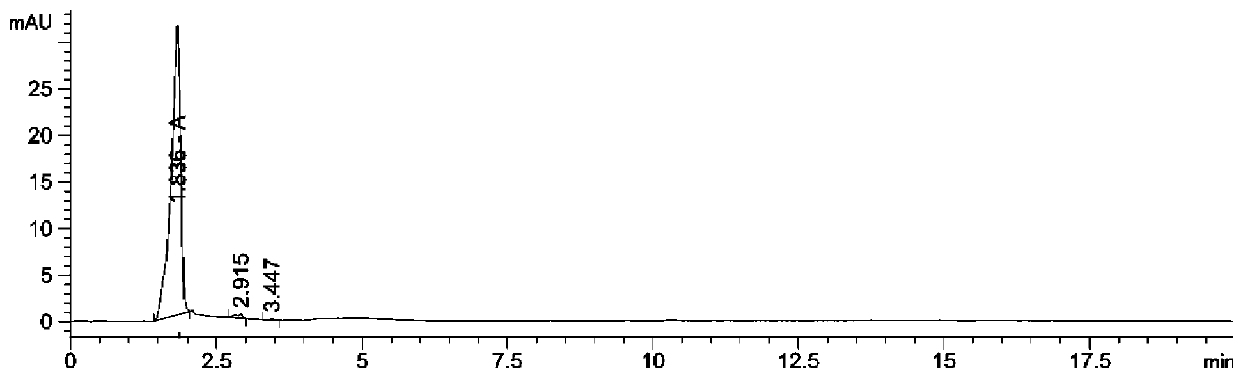
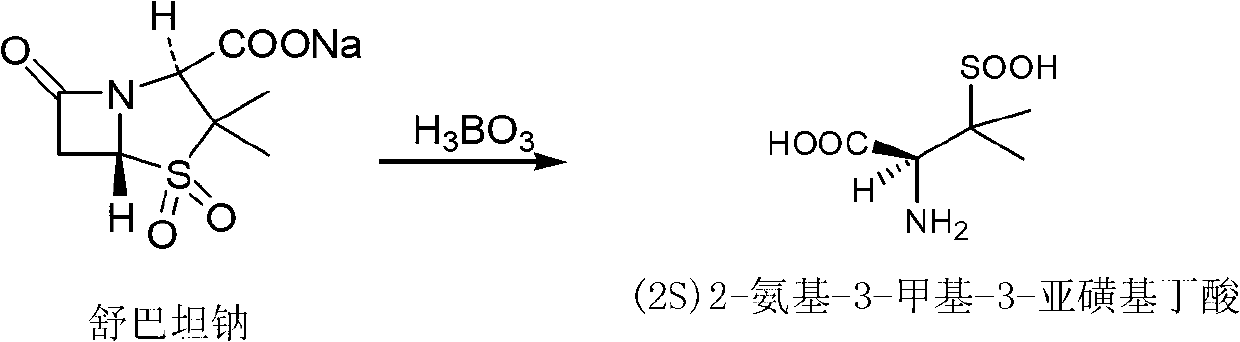
[0036] Put 150ml of ethanol and 15g of sodium hydroxide into a 500ml flask, cool to below 20°C, add 23.3g of sulbactam acid, dissolve heat, control the temperature at 20-25°C, react for 2h, the reaction is over, cool to 10°C, stir 1h, filter, rinse with appropriate amount of ethanol, dry under reduced pressure at 20-30℃ for 3h to obtain (2S)2-amino-3-methyl-3-sulfinylbutyric acid (also known as sulbactam penicillamine) 15.5g, 98.6% purity (see figure 1 , Where peak A is (2S)2-amino-3-methyl-3-sulfinylbutyric acid), the yield is 85.5%.
Embodiment 2
[0038] Put 300ml of ethanol and 20g of potassium hydroxide into a 500ml flask, lower the temperature to below 20°C, add 23.3g of sulbactam acid, dissolve exothermic, control the temperature at 20-30°C, react for 4h, the reaction is over, cool to 10°C, stir 1h, filter, rinse with appropriate amount of ethanol, dry under reduced pressure at 20-30℃ for 3h to obtain (2S)2-amino-3-methyl-3-sulfinylbutyric acid (also known as sulbactam penicillamine) 16.0g, purity 96%, yield 88.4%.
Embodiment 3
[0040] Put 150ml of ethanol and 16g of sodium hydroxide into a 500ml flask, lower the temperature to below 20°C, add 25.5g of sulbactam sodium, dissolve heat, control the temperature at 20-25°C, react for 5h, the reaction is over, cool to 5°C, stir 1h, filter, rinse with appropriate amount of ethanol, dry under reduced pressure at 20-30℃ for 3h to obtain (2S)2-amino-3-methyl-3-sulfinylbutyric acid (also known as sulbactam penicillamine) 16.0g, purity 96%, yield 88.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com