Method for utilizing o-chlorocyclohexanol to prepare cyclohexene oxide by cyclization
A technology of o-chlorocyclohexanol and epoxycyclohexane, which is applied in the field of organic chemical synthesis, can solve problems such as inability to produce large quantities of products, fail to meet industrial needs, and cumbersome process routes, and achieve practical and easy processes and production The effect of cost reduction and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0022] The preparation of the first step solid base
[0023] Take 8g of activated carbon, impregnate with equal volume of the prepared 4mol / L potassium carbonate solution, impregnate at 40°C for 4h, dry at 100-120°C, then roast in a muffle furnace at 500°C for 5h, cool to room temperature and grind , That is, the loading of potassium carbonate is 40%.
[0024] The preparation of the 2nd step epoxycyclohexane
[0025] Add 4g of the above-mentioned solid base, 5g of o-chlorocyclohexanol, and 20mL of absolute ethanol into a 50mL round-bottomed flask, install a condenser tube, heat and stir in an oil bath at 75°C for 2 hours, and analyze the product by gas chromatography, epoxycyclohexane The yield can reach 80.36%, and the selectivity to epoxycyclohexane can reach 98.67%.
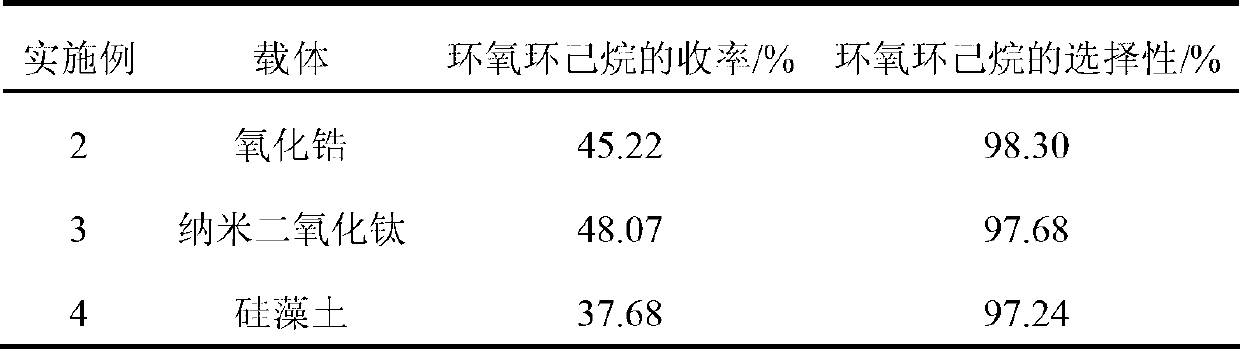
Embodiment example 2~4
[0027] Except that the following carriers are different, the others are the same as Example 1, and the carriers shown in Table 1 are used respectively.
[0028] Table 1
[0029]
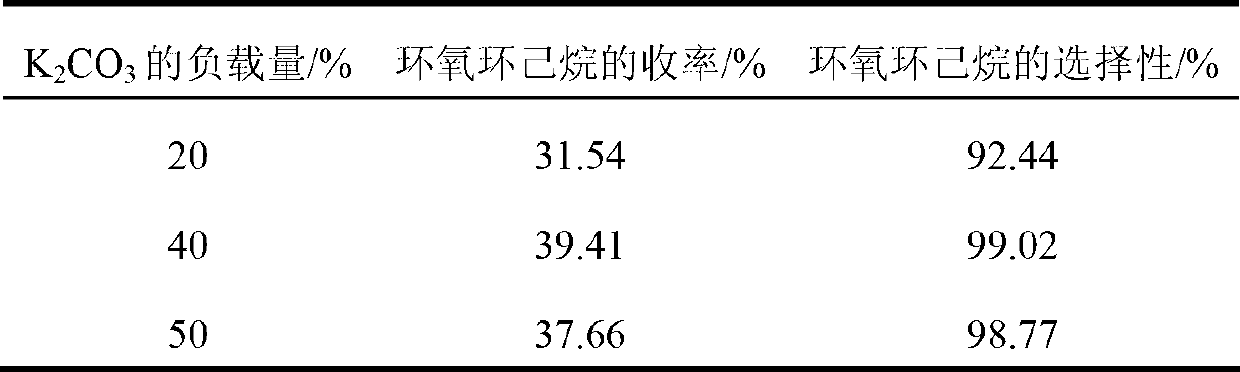
Embodiment example 5
[0031] Into a 50mL round bottom flask, add 1g loading capacity of 20, 40, 50% K 2 CO 3 impregnated K 2 CO 3 / C solid base (the preparation method of the solid base is the same as in Example 1 except for the loading capacity), 5g of o-chlorocyclohexanol, 20mL of ethanol with a condenser tube, heated and stirred in a 75°C oil bath for 2h, and the product was analyzed by gas chromatography Analysis, the yield and selectivity of epoxycyclohexane are as shown in table 2.
[0032] Table 2
[0033]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com