Method for preparing surface modification layer of lithium-rich material based on buffer solution system
A lithium-rich material and buffer solution technology, applied in structural parts, electrical components, battery electrodes, etc., can solve problems such as difficult industrialization and inability to achieve uniform coating, and achieve easy operation, non-toxic and harmless cost, and large discharge The effect of capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
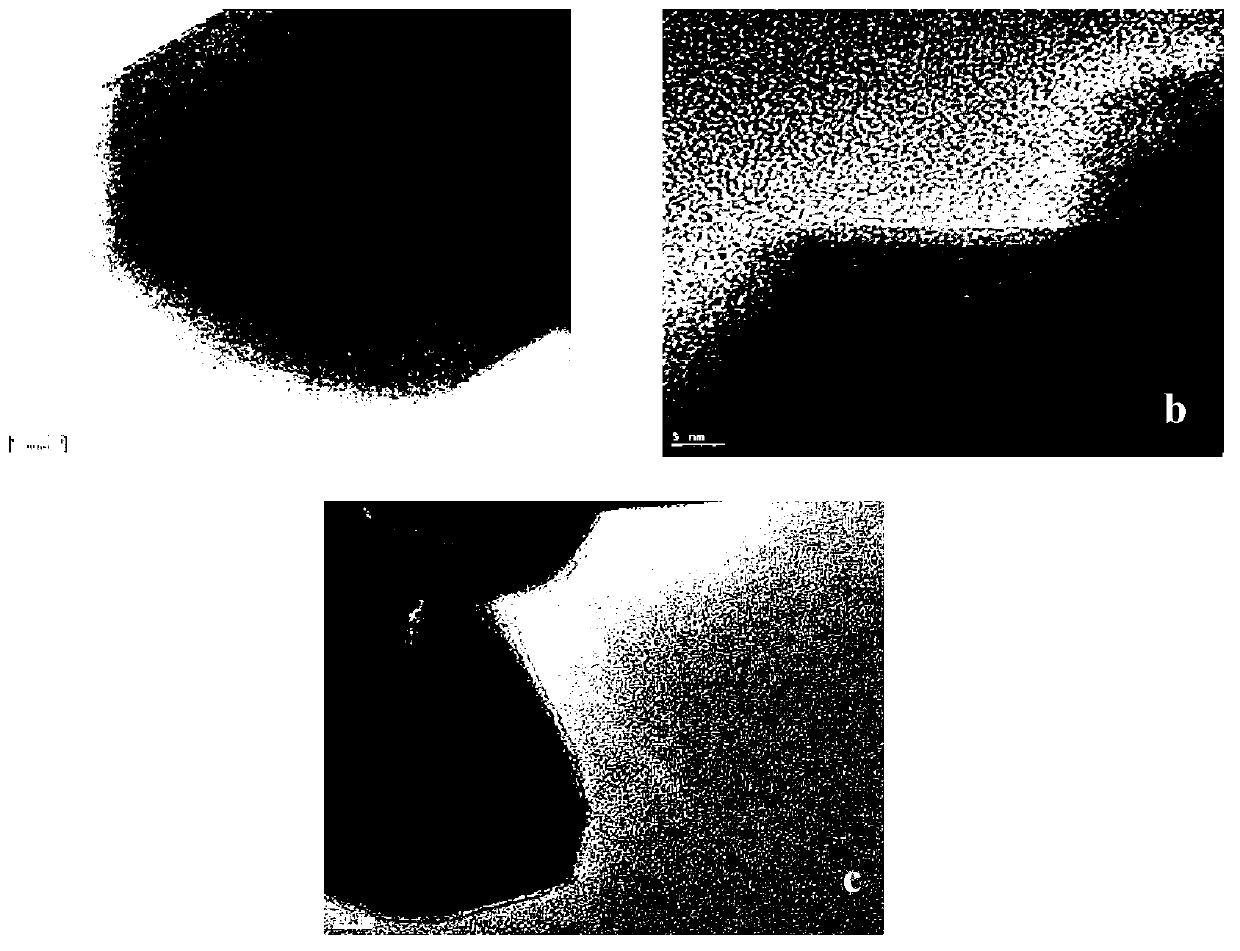
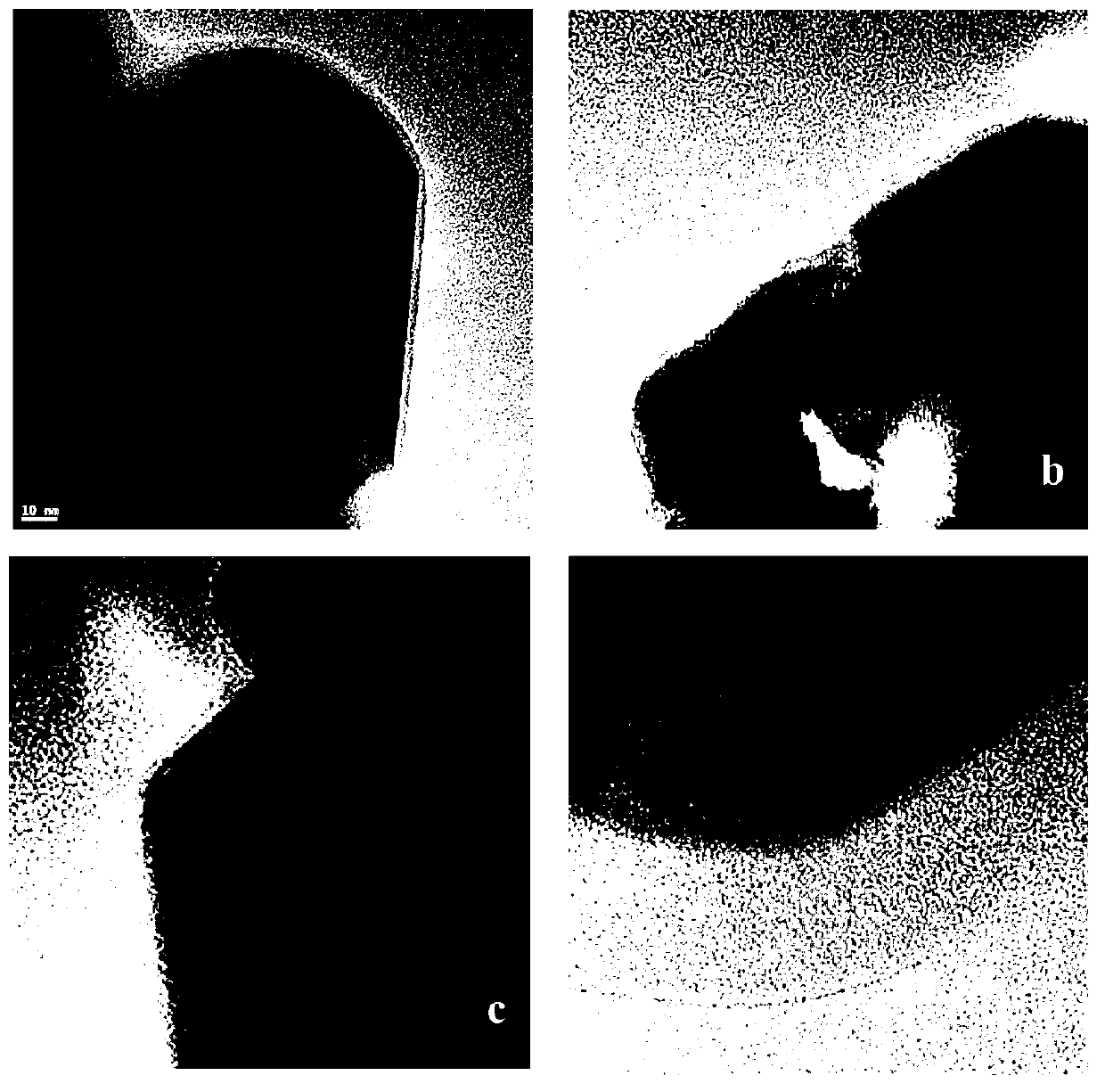
Image
Examples
Embodiment 1
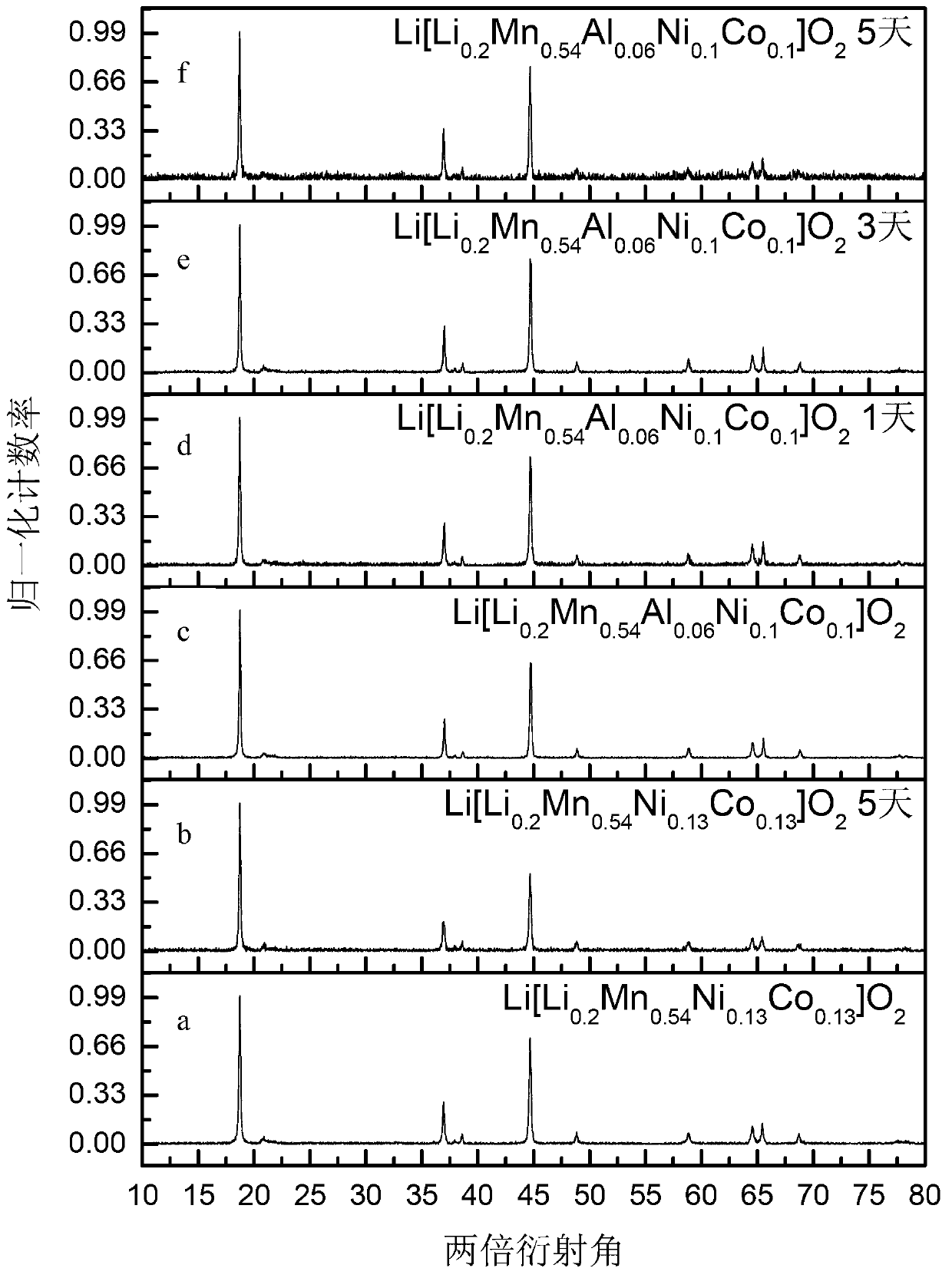
[0026] (1) Preparation of lithium-rich materials: according to Li[Li 0.2 mn 0.54 Ni 0.13 co 0.13 ]O 2The proportion of Ni, Co, Mn, Li in the preparation of metal acetate mixed solution; the mixed solution of citric acid, ethylene glycol as a complexing agent added to the metal acetate solution; the final mixed solution at 80 ° C Rotary evaporation to form a sol; the sol was dried in a vacuum oven at 150°C for 6 hours; finally, it was kept at 450°C for 6 hours in an air atmosphere in a tube furnace, and then kept at 850°C for 15 hours to obtain the final product Li[Li 0.2 mn 0.54 Ni 0.13 co 0.13 ]O 2 ;
[0027] (2) The prepared Li[Li 0.2 mn 0.54 Ni 0.13 co 0.13 ]O 2 Sieve (aperture 0.045mm sieve);
[0028] (3) Take 0.3g of the sieved sample and place it in a beaker, add 50ml of 0.2M PBS pH=7 standard buffer solution;
[0029] (4) Magnetic stirring at room temperature (speed 250rpm), reaction time 5 days;
[0030] (5) Wash the sample after the reaction with deion...
Embodiment 2
[0034] (1) Preparation of lithium-rich materials: according to Li[Li 0.2 mn 0.54 Ni 0.13 co 0.13 ]O 2 The proportion of Ni, Co, Mn, Li in the preparation of metal acetate mixed solution; the mixed solution of citric acid, ethylene glycol as a complexing agent added to the metal acetate solution; the final mixed solution at 80 ° C steamed to form a sol; the sol was dried in a vacuum oven at 150°C for 6 hours; finally, the final product Li[Li 0.2 mn 0.54 Ni 0.13 co 0.13 ]O 2 ;
[0035] (2) The prepared Li[Li 0.2 mn 0.54 Ni 0.13 co 0.13 ]O 2 Sieve (aperture 0.045mm sieve);
[0036] (3) Take 0.3g of the sieved sample and put it in a beaker, add 50ml of 0.1M PBS pH=5.7 standard buffer solution;
[0037] (4) Magnetic stirring at room temperature (speed 500rpm), reaction time 5 days;
[0038] (5) Wash the sample after the reaction with deionized water repeatedly, and dry it in a vacuum oven at 90° C. for 5 hours after suction filtration;
[0039] (6) Heat the above m...
Embodiment 3
[0042] (1) Preparation of lithium-rich materials: according to Li[Li 0.2 Ni 0.1 co 0.1 al 0.06 mn 0.54 ]O 2 The proportion of Ni, Co, Mn, Al, Li in the preparation of metal acetate mixed solution; the mixed solution of citric acid, ethylene glycol as a complexing agent added to the metal acetate solution; the final mixed solution at 80 ℃ The sol was formed by rotary evaporation; the sol was dried in a vacuum oven at 150°C for 6 hours; finally, the final product Li[Li 0.2 Ni 0.1 co 0.1 al 0.06 mn 0.54 ]O 2 ;
[0043] (2) The prepared Li[Li 0.2 Ni 0.1 co 0.1 al 0.06 mn 0.54 ]O 2 Sieve (aperture 0.074mm sieve);
[0044] (3) Take 0.3g of the sieved sample and put it in a beaker, add 50ml of 0.1M PBS pH=7 standard buffer solution;
[0045] (4) Magnetic stirring at room temperature (rotational speed 250rpm), the reaction time is 1 day, 3 days and 5 days respectively;
[0046] (5) Wash the sample after the reaction with deionized water repeatedly, and dry it in a v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com