Esterification reaction-pervaporation membrane separation integrated method for producing methyl 3-hydroxypropionate
A technology of pervaporation membrane and hydroxypropionic acid, which is applied in chemical instruments and methods, separation/purification of carboxylic acid esters, preparation of organic compounds, etc., and can solve the problem of combined membrane technology that has not involved 3-hydroxypropionate reaction, etc. problems, to achieve the effects of small membrane area, increased yield, and improved production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
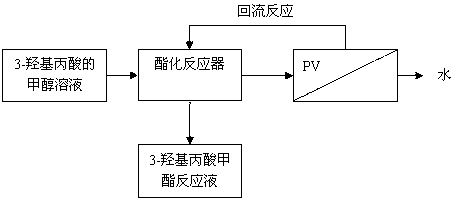
[0023] see figure 1 , 3-Hydroxypropionic acid methylation reaction-pervaporation membrane separation integrated process flow is:
[0024] Preparation of binary reaction liquid of 3-hydroxypropionic acid and methanol→esterification reaction→pervaporation membrane separation→termination of methyl esterification reaction. The method of the invention starts from the 3-hydroxypropionic acid raw material, undergoes an esterification reaction-pervaporation membrane separation and integration process, and finally obtains high-purity methyl 3-hydroxypropionate liquid for the production of 3-hydroxypropionic acid.
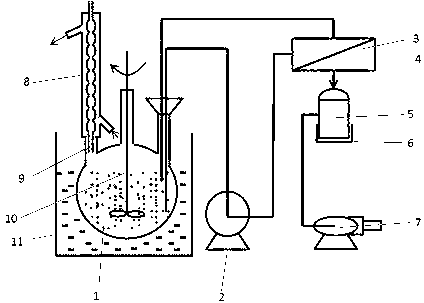
[0025] exist figure 2 The esterification reaction-pervaporation membrane separation is performed in the experimental setup of the shown integrated process. Take 80 mL of methanol (density 0.792 g / mL), inject it into a three-necked flask with a stirrer and a reflux condenser, turn on the stirrer, and then inject 82 g of 3-hydroxypropionic acid (methanol and 3-hydroxypropio...
Embodiment 2
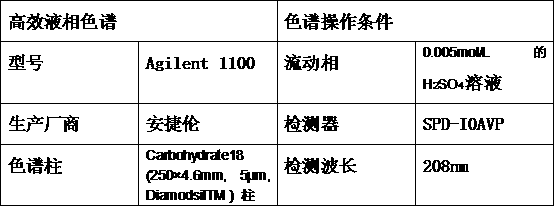
[0030]According to the operation of Example 1, wherein, only changing the effective membrane area of the pervaporation membrane and the reaction liquid volume ratio is 0.063cm -1 , the vacuum on the downstream side of the membrane was 600pa. When the methyl esterification reaction ended, HPLC analysis showed that 93.86% of the 3-hydroxypropionic acid in the reaction solution was converted into 3-hydroxypropionic acid methyl ester, and 152mL of light yellow 3-hydroxypropionic acid was obtained. Methyl propionate solution.
Embodiment 3
[0032] According to the operation of Example 1, wherein only the mass ratio of methanol to 3-hydroxypropionic acid is changed to 1.04, and the vacuum degree downstream of the membrane is 650 Pa, HPLC experiments show that 97.2% of 3-hydroxypropionic acid in the reaction solution is converted into 3-hydroxypropionic acid acid methyl ester. 149 ml of a pale yellow methyl 3-hydroxypropionate solution are obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com