A semi-quantitative detection method of chlorine bromide iodide ion using indicator displacement reaction
A displacement reaction, iodide ion technology, applied in the field of semi-quantitative detection of chlorine bromide or iodide ion, can solve the problems of difficult color distinction, impossible quantitative analysis of halogen ions, etc., to achieve the effect of improving sensitivity and enhancing color change
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0039] Establishment of standard "fingerprint" library:
[0040] Specifically:
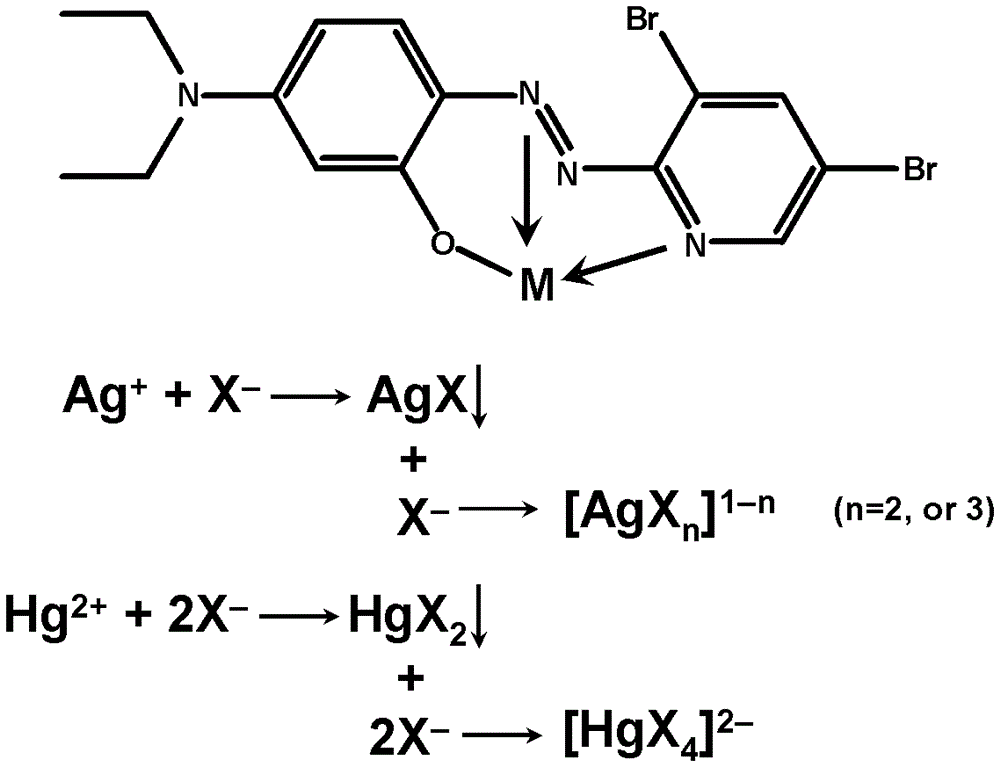
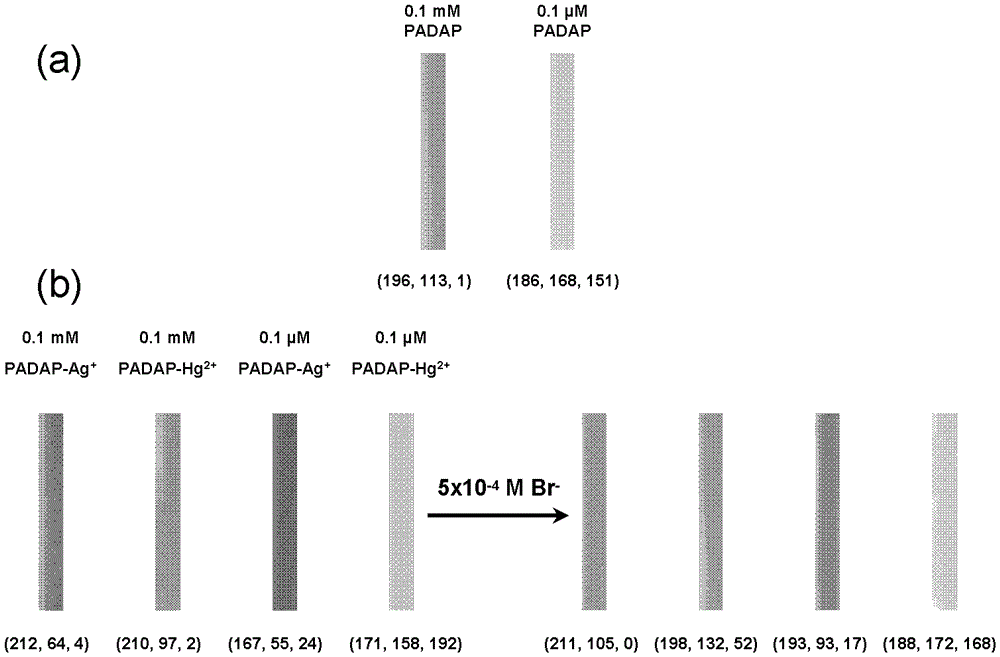
[0041] 1) Metal ion chromogen and metal ion chelation: ①Add 20 μL of 0.01mol / L silver nitrate aqueous solution to 1.98mL of 0.1mM3,5-Br2-PADAP ([2-(3,5)-dibromo-2-pyridine Nitrogen]-5-diethylaminophenol) in the alcohol-water mixed solution (volume ratio 1:1) of the metal ion developer, mix well; -Br2-PADAP metal ion chromogen in the alcohol-water mixed solution (volume ratio 1:1), fully mix; ③20μL 0.01mmol / L silver nitrate aqueous solution is added to 1.98mL0.1μM3, 5-Br2-PADAP metal ion development In the alcohol-water mixed solution of the colorant (volume ratio 1:1), mix thoroughly; ④Add 20μL of 0.01mmol / L mercuric chloride aqueous solution to 1.98mL of 0.1μM 3,5-Br2-PADAP metal ion chromogen in alcohol-water mixture solution (volume ratio 1:1), mix thoroughly;
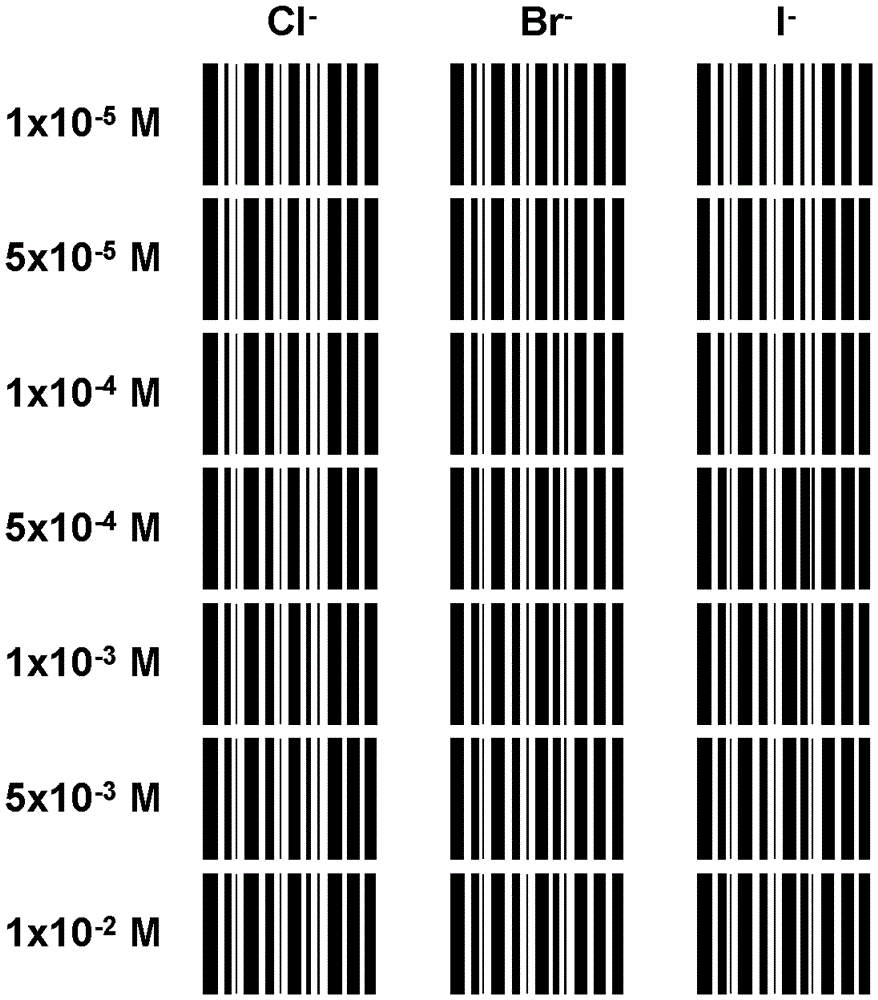
[0042] 2) Displacement reaction of the indicator: add 1x10 -5 , 5x10 -5 , 1x10 -4 , 5x10 -4 , 1x10 -3 , 5x10 -3 and 1x10 -2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com