Lithium iron conductive complex modified lithium iron phosphate anode material and preparation method thereof
A lithium iron phosphate, cathode material technology, applied in battery electrodes, circuits, electrical components, etc., can solve problems such as being unable to meet the requirements of long life of electric vehicles, reducing battery volume specific power, and deteriorating surface stability. The effect of lithium ion diffusivity, improving mechanical properties and increasing bond strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
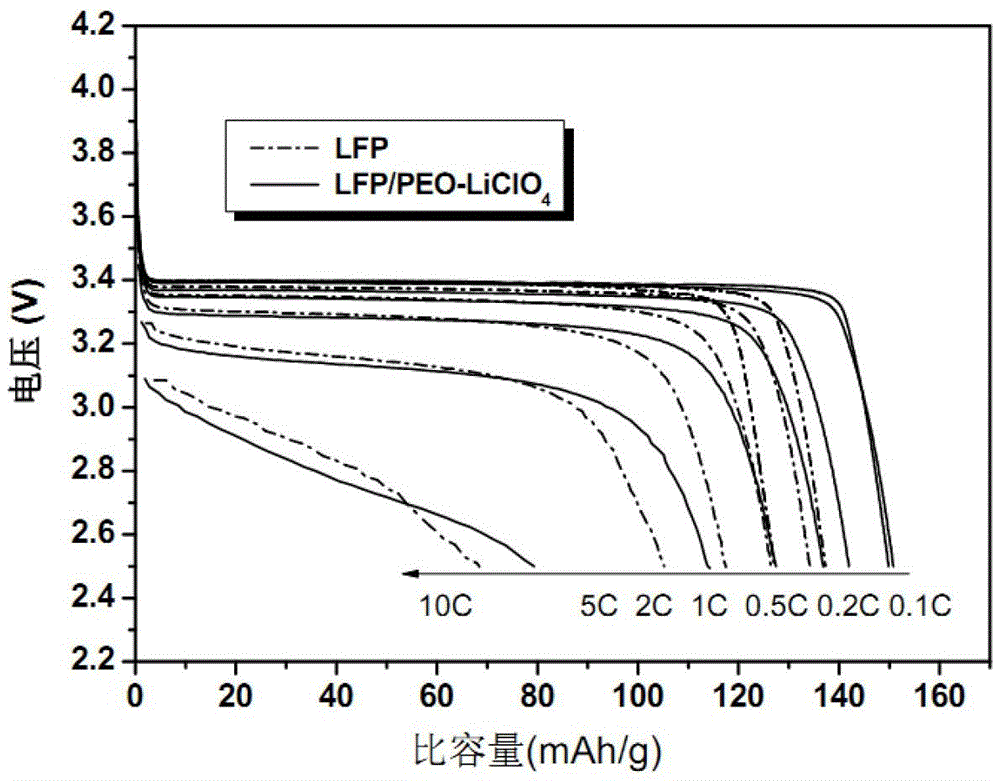
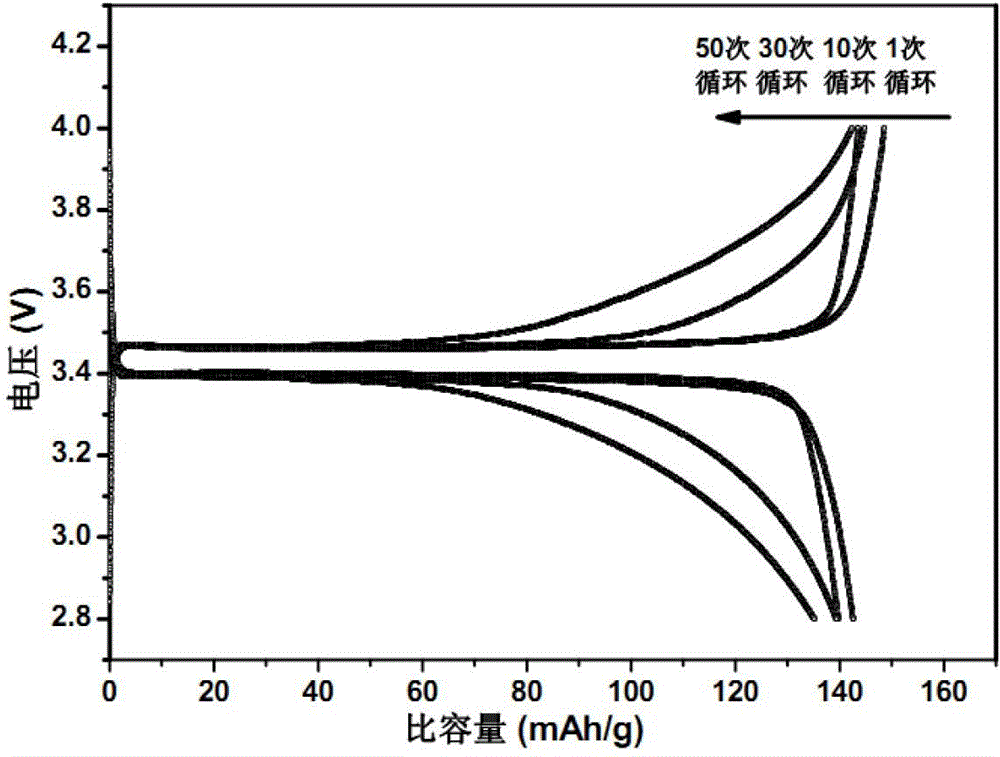
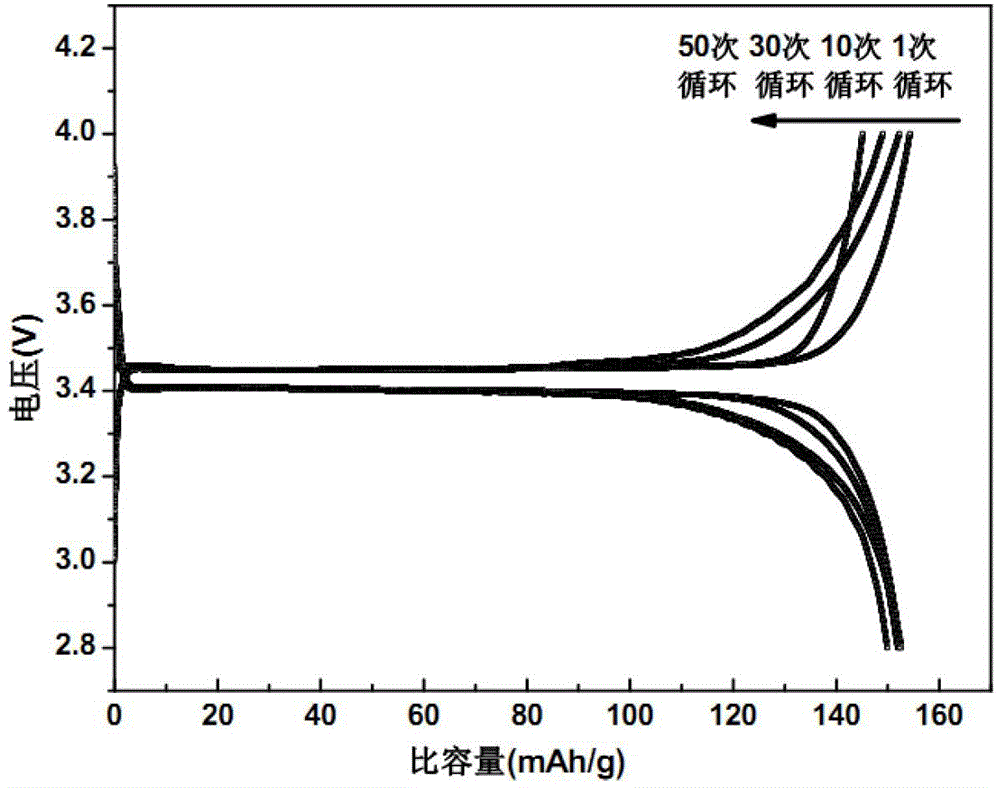
[0019] 1g of polyethylene oxide (PEO) was dissolved in anhydrous acetonitrile to form a 5% solution by mass, and LiClO was added 4 (Its molar ratio with PEO is PEO repeating unit / Li + =8 / 1), stirred at room temperature for 1 hour, and dried the solvent to obtain PEO / LiClO 4 Lithium ion conducting complex. 1.2gPEO / LiClO 4 The lithium ion conductive complex and 12 g of lithium iron phosphate powder were ultrasonically oscillated in anhydrous acetonitrile for 60 minutes, and the solvent was dried to obtain a lithium iron phosphate positive electrode material modified by the lithium ion conductive complex.
[0020] After mixing lithium iron phosphate cathode material powder (active material) modified by lithium ion conductive complex with acetylene black (conductive agent) and PTFE (binder) according to the mass ratio of 75:20:5, drop an appropriate amount of Stir the isopropanol solvent evenly, and press it into a uniform film with a film laminator. After fully drying at 120°...
Embodiment 2
[0023] 1g polyacrylonitrile (PAN) is dissolved in the mixed solvent of anhydrous N,N-dimethylformamide and N,N-dimethylacetamide to form a solution with a mass percentage of 15%, adding LiAsF 6 (Its molar ratio with PAN is PAN repeating unit / Li +=5 / 1), stirred at room temperature for 2 hours, and dried the solvent to obtain PAN / LiAsF 6 Lithium ion conducting complex. 1.2g of the lithium ion conductive complex and 6g of lithium iron phosphate powder were ultrasonically oscillated for 40 minutes in a mixed solvent of anhydrous N,N-dimethylformamide and N,N-dimethylacetamide, and the solvent was dried That is, the lithium iron phosphate positive electrode material composite positive electrode material modified by the lithium ion conductive complex is obtained.
Embodiment 3
[0025] 1g of polymethyl methacrylate (PMMA) was dissolved in anhydrous N,N-dimethylacetamide to form a 30% solution by mass, and LiPF was added 6 (Its mol ratio with PMMA is PMMA repeating unit / Li + =10 / 1), stirred at room temperature for 5 hours to obtain PMMA / LiPF 6 Lithium ion conducting complex. 1.2g of the lithium ion conductive complex and 2.4g of lithium iron phosphate powder were ultrasonically oscillated in anhydrous N, N-dimethylacetamide for 60 minutes, and the solvent was dried to obtain phosphoric acid modified by the lithium ion conductive complex. Iron-lithium cathode material composite cathode material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com