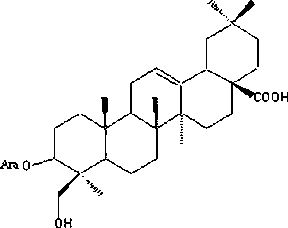
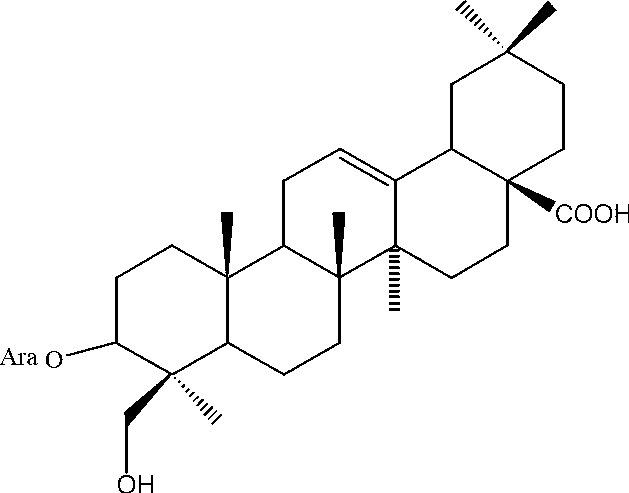
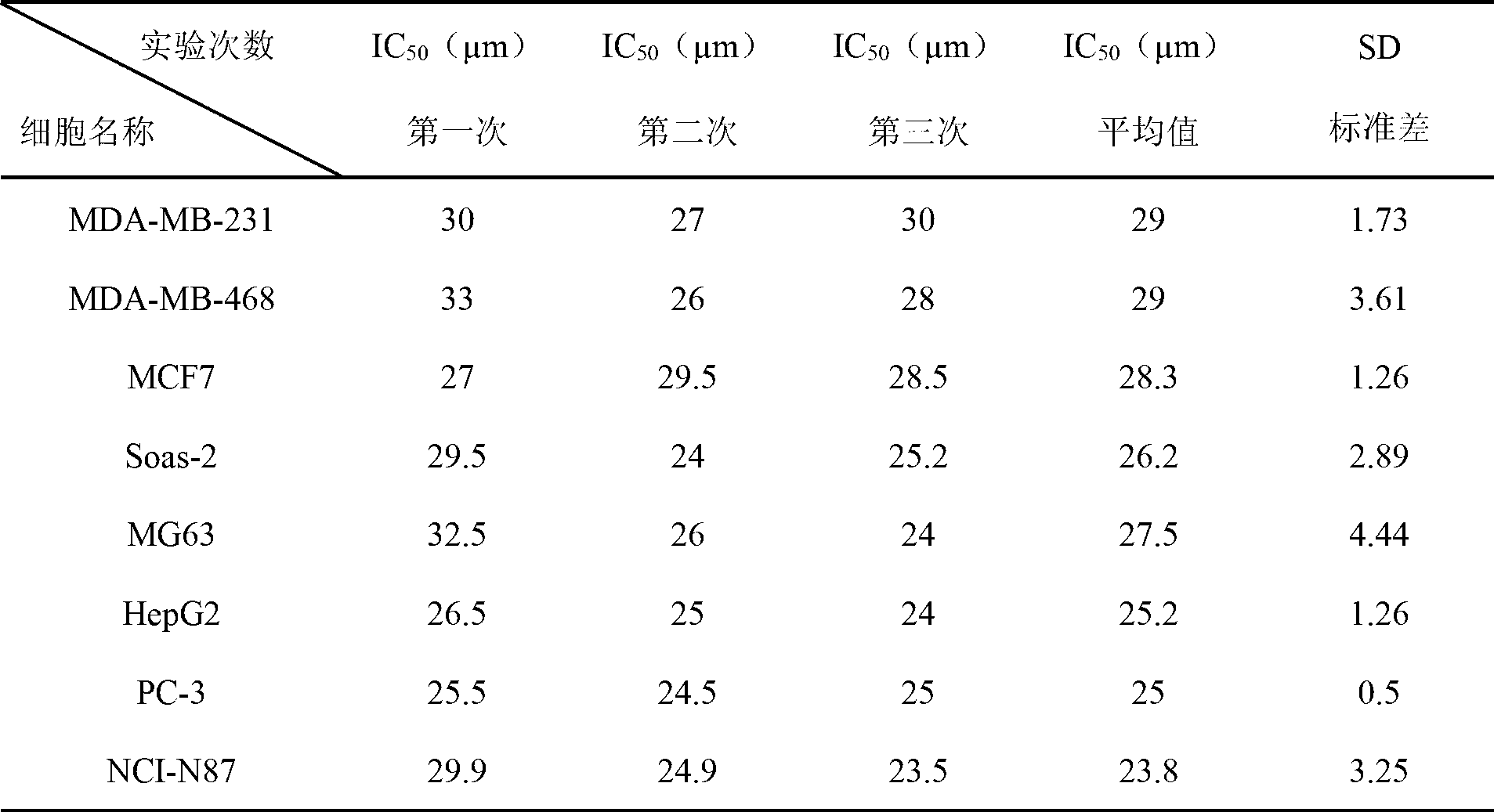
Antineoplastic application of Hederagenin-3-O-arabinopyranoside
An anti-tumor and anti-tumor drug technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation of embodiment 1 Hederagenin-3-O-arabinopyranoside
[0039] Weigh 10kg of tiger palm herb crude powder, add 6 times the amount of methanol, cold soak at room temperature 5 times, each time not less than 24h, filter, combine the filtrate, recover the filtrate under reduced pressure, vacuum dry, crush, and obtain dry extract 1 . Weigh the above dry extract 1, add ethyl acetate equivalent to 5 times the amount of extract, reflux extraction four times, two hours for the first time, one hour for the second, third, and fourth times, filter, combine the filtrate, and extract Concentrate under reduced pressure and dry in vacuo to obtain ethyl acetate extracts. Take the extracted part of ethyl acetate, mix the sample with silica gel (80-100 mesh), and then perform silica gel column chromatography (200-300 mesh), and use chloroform, chloroform-methanol (9:1), chloroform-methanol (8:2), Chloroform-methanol (7:3) and chloroform-methanol (6:4) were used for gradient...
Embodiment 2
[0041] Take 5.0g of Hederagenin-3-O-arabinopyranoside, 10.0g of starch, and 7.85g of microcrystalline cellulose, pass through an 80-mesh sieve, mix well, add 3% hydroxypropyl cellulose and stir to make a soft material, pass through a 16-mesh sieve Dry the wet granules below 50°C, pass the dry granules through a 16-mesh sieve, add 1.0 g of croscarmellose sodium and 0.75 g of magnesium stearate, mix well, and press into 100 tablets. Each tablet weighs 0.25g, contains Hederagenin-3-O-arabinopyranoside 50mg, and can be used for the treatment of gastric cancer.
Embodiment 3
[0043] Grind Hederagenin-3-O-arabinopyranoside into powder, pass through an 80-mesh sieve, mix 5.0 g with 11.5 g of starch and 8.5 g of micronized silicon, sieve, pack into capsules, and make 100 capsules. Each capsule weighs 0.25g, contains Hederagenin-3-O-arabinopyranoside 50mg, and can be used for the treatment of liver cancer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com