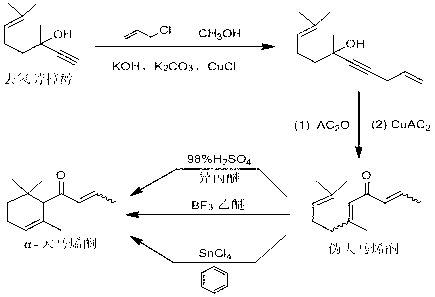
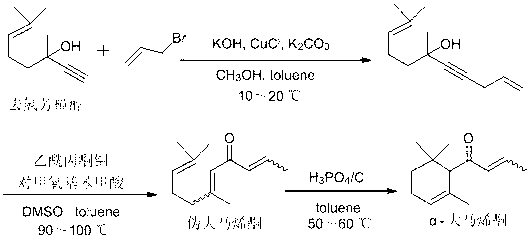
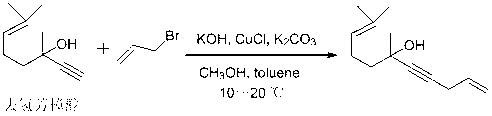Preparation method of alpha-damascenone perfume
A damascenone fragrance and technology of damascenone, which is applied in the field of fragrance fine chemicals, can solve the problems of low α-damascenone yield, high cost, short route, etc., and achieve high reaction yield and easy operation , the effect of short route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Preparation of 6,10-Dimethylundecane-1,9-dien-4-yn-6-ol
[0017]
[0018] In a suitable reaction bottle, control the temperature at 10-20 °C, add methanol (746 ml) into the suspension of KOH (162 g) in toluene (720 ml), control the temperature at 10-20 °C and stir for 15 minutes. Control the temperature at 10-20 °C, mix CuCl (16 g) and K 2 CO 3 (22 g) was added into the reaction flask, and the temperature was controlled at 10-20°C and stirred for 15 minutes. Control the temperature at 10-20 °C, add dehydrolinalool (304 g) into the reaction flask, after the addition is complete, stir for 15 minutes, then, control the temperature at 10-20 °C, add 3-bromopropene (258 g) Slowly add it dropwise into the reaction flask, and after the addition is complete, control the temperature at 10-20°C for the reaction. Gas chromatography (GC) is used to track the reaction. After the reaction of the raw materials is completed, an appropriate amount of dilute hydrochloric acid is add...
Embodiment 2
[0021] Preparation of 6,10-dimethylundecane-2,5,9-trien-4-one or pseudodamascenone
[0022]
[0023] Add molybdenum acetylacetonate (51 g), dimethyl sulfoxide (43 g), p-methoxybenzoic acid (47 g) and toluene (1198 ml) respectively into a suitable reaction vessel, stir and heat to 100 °C, then add 6,10-Dimethylundecane-1,9-dien-4-yn-6-ol (150 g) was reacted at a temperature of 100 °C. Gas chromatography (GC) monitors until the reaction of the raw materials is complete, the mother liquor is cooled to room temperature, filtered, washed with water, the organic phase is separated, dried over anhydrous sodium sulfate, and precipitated to obtain a crude product with a yield of 92%.
[0024] ESI MS m / z :193 [M+H] + ; 1 H NMR (400 MHz, CDCl 3 ): δ = 6.84-6.74 (m, 1H), 6.15-6.10 (m, 2H), 5.13-5.05 (m, 1H), 2.72-1.84 (m, 10H), 1.65-1.58 (m, 6H).
Embodiment 3
[0026] Preparation of 6,10-dimethylundecane-2,5,9-trien-4-one or pseudodamascenone
[0027]
[0028] Add molybdenum acetylacetonate (25 g), dimethyl sulfoxide (30 g), p-methoxybenzoic acid (12 g) and toluene (868 ml) respectively into a suitable reaction vessel, stir and heat to 100 °C, then add 6,10-Dimethylundecane-1,9-dien-4-yn-6-ol (150 g) was reacted at a temperature of 90 °C. Gas chromatography (GC) monitors that the reaction of the raw materials is complete, the mother liquor is cooled to room temperature, filtered, washed with water, the organic phase is separated, dried over anhydrous sodium sulfate, and the crude product is obtained by precipitation with a yield of 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com