Natural compound and preparation method and application thereof
A compound and drug technology, applied in the field of natural compounds and their preparation, can solve the problems of delay in the research and development of anti-tumor drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of compound shown in embodiment 1, formula I
[0048] a. Solid fermentation of strain Doratomyces sp.
[0049] Activation of the strain Doratomyces.sp. PDA medium: 200g of potatoes, 20g of glucose, 15g of agar, 1000mL of purified water, sterilized by high-pressure steam at 121°C for 30 minutes, made into a test tube slant, picked mycelium and inoculated it on the test tube slant, and cultured at 25°C for 10 days.
[0050] Solid state fermentation of strain Doratomyces sp. Preparation of rice culture medium (add 800g rice and 1200mL purified water into ten 500mL Erlenmeyer flasks, soak overnight, sterilize by high-pressure steam at 121°C for 30 minutes, and cool for later use); pick mycelium from the inclined surface of the test tube in Prepare a bacterial suspension in a test tube filled with sterile water, and prepare the prepared bacterial suspension (spore concentration of 1×10 6 Individuals / mL) 5 mL was inoculated on the rice medium, and fermented...
Embodiment 2
[0062] The structural characterization of the compound shown in embodiment 2, formula I
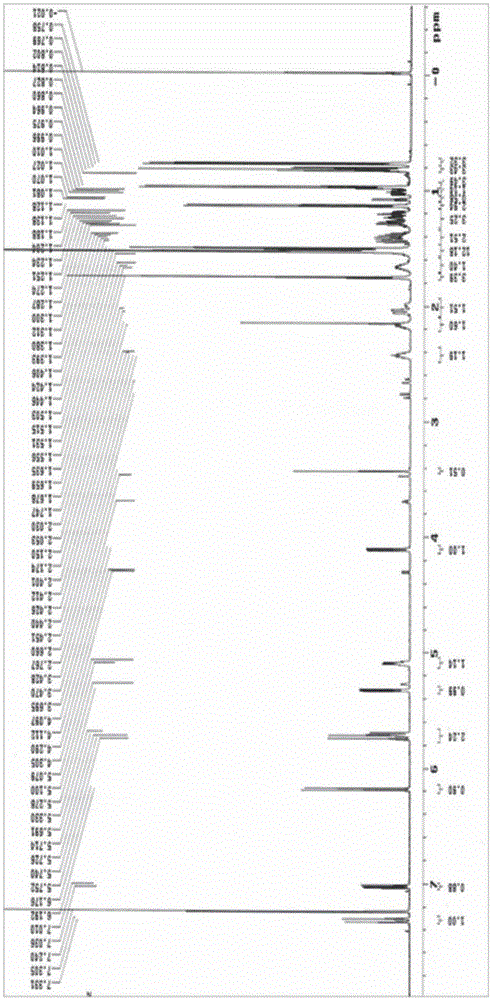
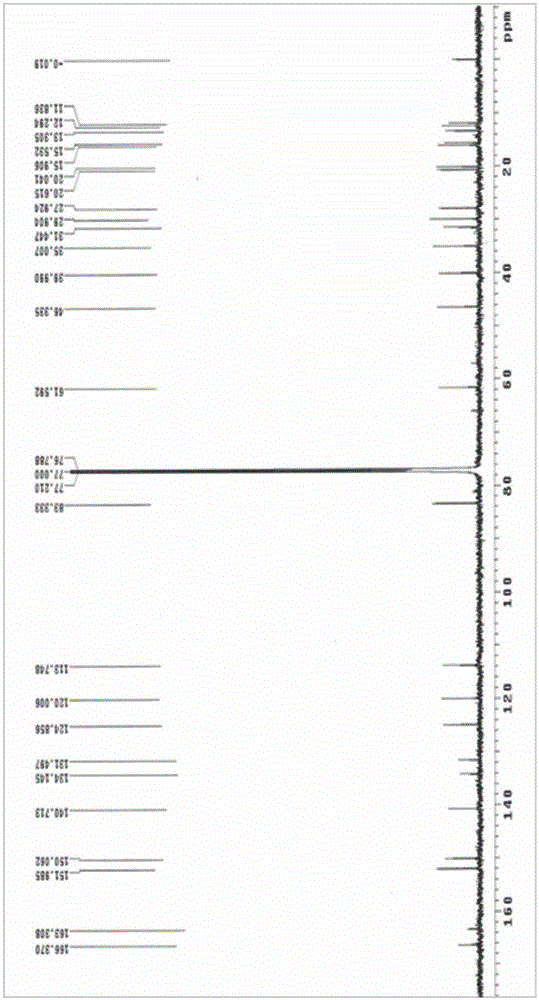
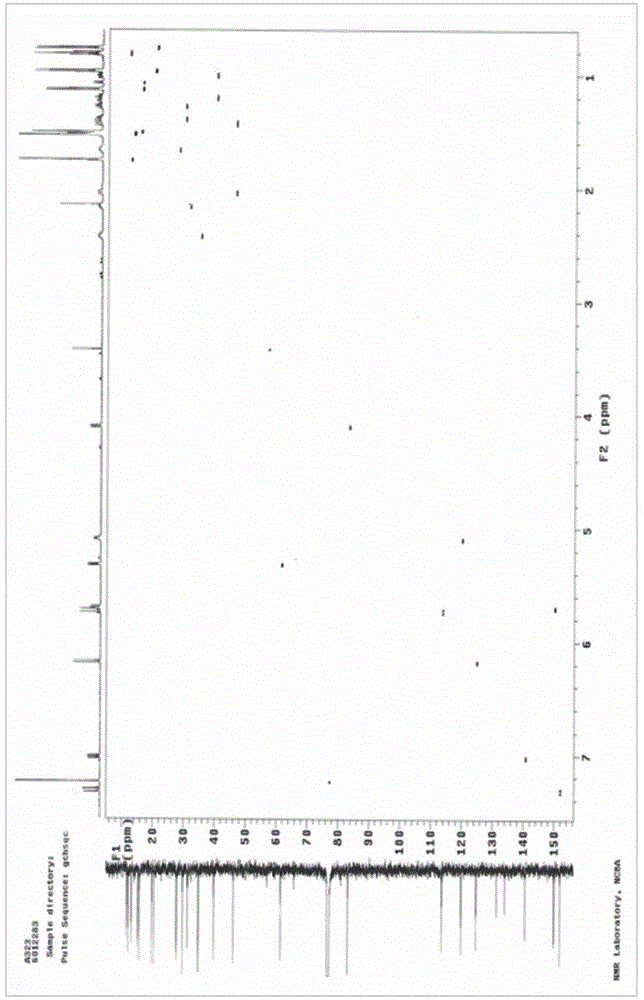
[0063] The compound shown in formula I prepared in Example 1 was analyzed by high-resolution mass spectrometry (HRESIMS), infrared spectrum (IR), nuclear magnetic resonance spectrum (NMR), circular dichroism (CD) and rotational spectroscopy (ORD), wherein high-resolution mass spectrometry The test was provided by the Mass Spectrometry Test Center of the Institute of Microbiology, Chinese Academy of Sciences (the test instrument model is Agilent Accurate-Mass-Q-TOF LC / MS 6520), and the infrared spectrum was tested by the School of Chemistry, Peking University (the test instrument model is NicoletMagna-IR 750), The NMR spectrum was tested by the Instrument Analysis and Test Center of the Academy of Military Medical Sciences (the test instrument model is Varian UNTTY INOVA 600), the circular dichroism spectrum is tested by the Institute of Toxicology and Drugs of the Academy of Military Medi...
Embodiment 3
[0074] Embodiment 3, the effect of the compound shown in formula I on tumor cell reproductive growth
[0075] Construction of cell screening models: lung cancer cells (A549), breast cancer cells (MCF-7), colon cancer cells (SW480, HCT116), cervical cancer cells (HeLa) and bladder cancer cells (T24) (the above cells were provided by the Chinese Academy of Sciences Microbiology Provided by Jiang Xuejun's research group of the Institute).
[0076] MTT method (MTT colorimetric method) was used to test the effect of the compound represented by formula I prepared in Example 1 on the reproductive growth of the above six tumor cells. In order to verify the dosage effect of several tumor cells on the compound, the compound shown in formula I prepared in Example 1 and the positive control drug cisplatin were prepared with DMSO (Dimethyl sulfoxide, DMSO) as a solvent to a concentration of The solutions to be tested were 123.76 μM, 61.88 μM, 30.94 μM, 15.47 μM, 7.74 μM, 3.87 μM, 1.93 μM ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com