Method of preparing of hydroxypropyl methacrylate (HPMA) - dexamethasone polymer
A polymer and solution technology, which can be used in drug combinations, pharmaceutical formulations, and medical preparations with inactive ingredients, etc., can solve problems such as constraints, achieve good biocompatibility, and reduce systemic side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
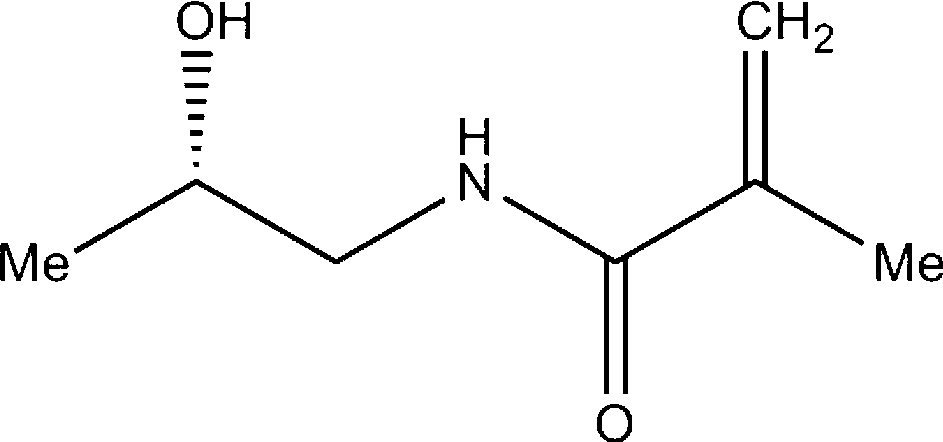
[0042] Embodiment 1 prepares HPMA
[0043] Dissolve 16ml (0.2mol) of 1-amino-2-propanol in 20ml of acetonitrile, stir at room temperature (20-25°C) for 10min, slowly drop methacryloyl chloride into the acetonitrile solution, and stir at room temperature for 1h. The reaction solution was kept at -35°C for 12 h until the white crystals were completely precipitated. Filter and wash the crystals with cold acetone. Dry under reduced pressure and recrystallize with a small amount of acetone. Yield: 63%. MS(ESI) m / z144(M+1).1H NMR(400MHz, CDCl 3):δ1.20and1.22(d,J=6.4Hz,3H),1.97(s,3H),3.18-3.21(m,1H),3.48-3.51(m,1H),3.95-3.96(m,1H ), 5.36(s,1H), 5.74(s,1H).
Embodiment 2
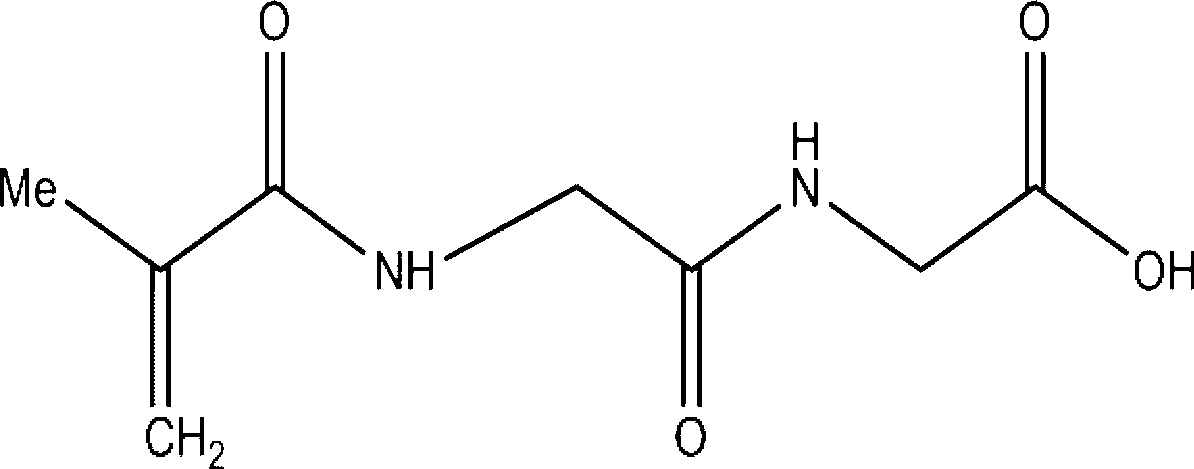
[0044] Embodiment 2 synthetic NAGGONP
[0045] Take 10ml of 0.5M NaOH (50.7mmol) aqueous solution to dissolve 5g of diglyglyceride (38mmol), then place it at 0°C and stir for 30min. Then slowly drop the mixture of 3.8m methacryloyl chloride (38mmol) and 10ml, 5M NaOH (50.7mmol) into the diglycyl peptide solution and keep stirring at 0°C for 30min. After completion, take out the solution, stir at room temperature for 2 hours, add HCl dropwise until the solution is neutral, stir slowly until a white solid precipitates, collect the solid and filter, wash with cold water, dry under reduced pressure, and recrystallize with a small amount of 50% ethanol solution . Yield: 30%. 1H NMR: (400MHz, DMSO-d6) δppm: 1.876(s, 3H), 3.766(d, 4H), 5.561(d, 2H), 8.132(t, 1H), 8.190(t, 1H), 12.594(s ,1H).Mp=175-205℃.
Embodiment 3
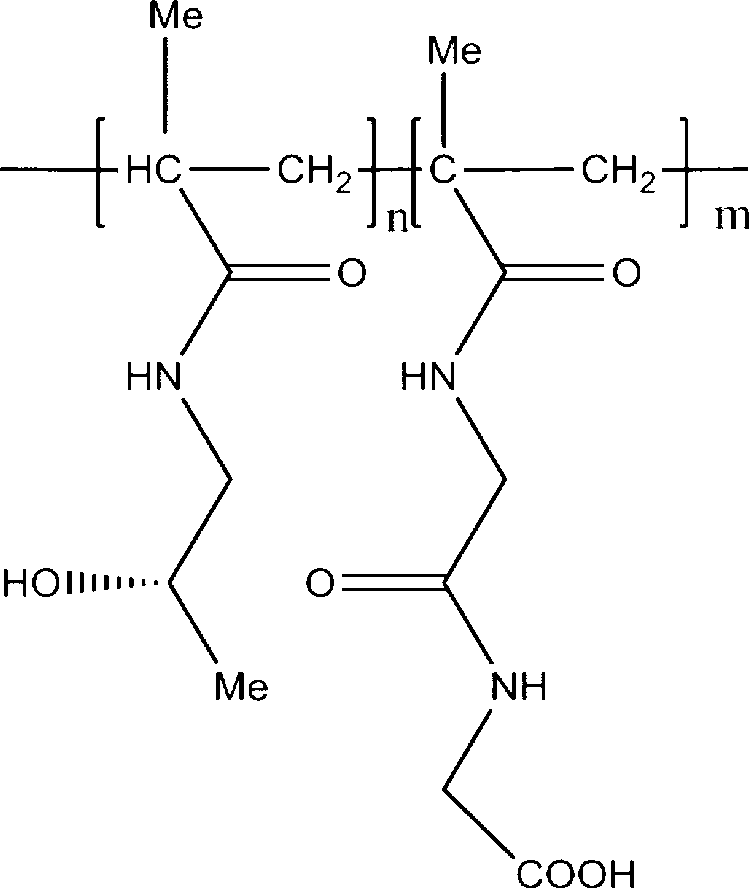
[0046] Embodiment 3 prepares HPMA polymer-NAGGONP conjugate
[0047] With 2,2-azobisisobutylcyanide (AIBN) as the initiator, 2.24g (15.6mmol) of HPMA monomer and 0.09g (0.55mmol) of AIBN were dissolved in 20ml of acetone, and 0.2g of NAGGONP (0.6mmol) was dissolved In 2mlDMSO, then the two solutions were mixed and miscible, and N was introduced into the solution 2 After 30 minutes, the system was sealed and heated and stirred in a water bath at 60°C for 18 hours. Stand still, filter after the precipitation is complete, wash the solid with cold acetone, dry under reduced pressure, dissolve in dichloromethane: methanol = 9:1, add cold acetone for recrystallization. Yield: 60%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com