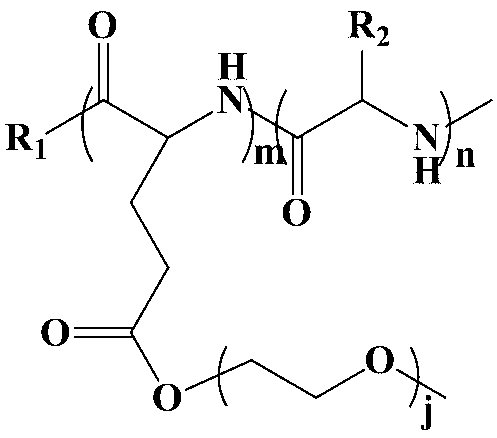
Poly (gamma-oligomerization ethylene glycol monomethyl ether-L-glutamic acid diethyl ester) - polyamino acid diblock copolymer and preparation method thereof
A technology of oligoethylene glycol and block copolymers, which is applied in the field of biodegradable polymer synthesis to achieve good biocompatibility and biodegradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Preparation of poly(γ-ethylene glycol monomethyl ether-L-glutamate)-poly(γ-benzyl-L-glutamate) diblock copolymers with different number average molecular weights:
[0057] Weighing quality is 0.1156 g (0.0005 mol), 0.2312 g (0.001 mol), 1.1560 g (0.005 mol), 1.1560 g (0.005 mol), 1.1560 g (0.005 mol), 1.1560 g (0.005 mol), 1.1560 g ( 0.005 mol), 2.3120 g (0.01 mol), 4.6240 g (0.02 mol) of γ-ethylene glycol monomethyl ether-L-glutamate-N-inner carboxylic acid anhydride are put into 9 anhydrous reaction bottles, Then add 5 mL of anhydrous N,N-dimethylformamide to dissolve the monomer, respectively add 10.119 mg (0.0001 mol) of n-hexylamine under stirring, react under stirring at 25°C for 72 hours, and then add Masses 1.3162 g (0.005 mol), 1.3162 g (0.005 mol), 1.3162 g (0.005 mol), 5.2648 g (0.02 mol), 2.6324 g (0.01 mol), 0.2632 g (0.001 mol), 0.1316 g (0.0005 mol) , 1.3162 g (0.005 mol), 1.3162 g (0.005 mol) of γ-benzyl-L-glutamic acid ester-N-internal carboxylic acid ...
Embodiment 2
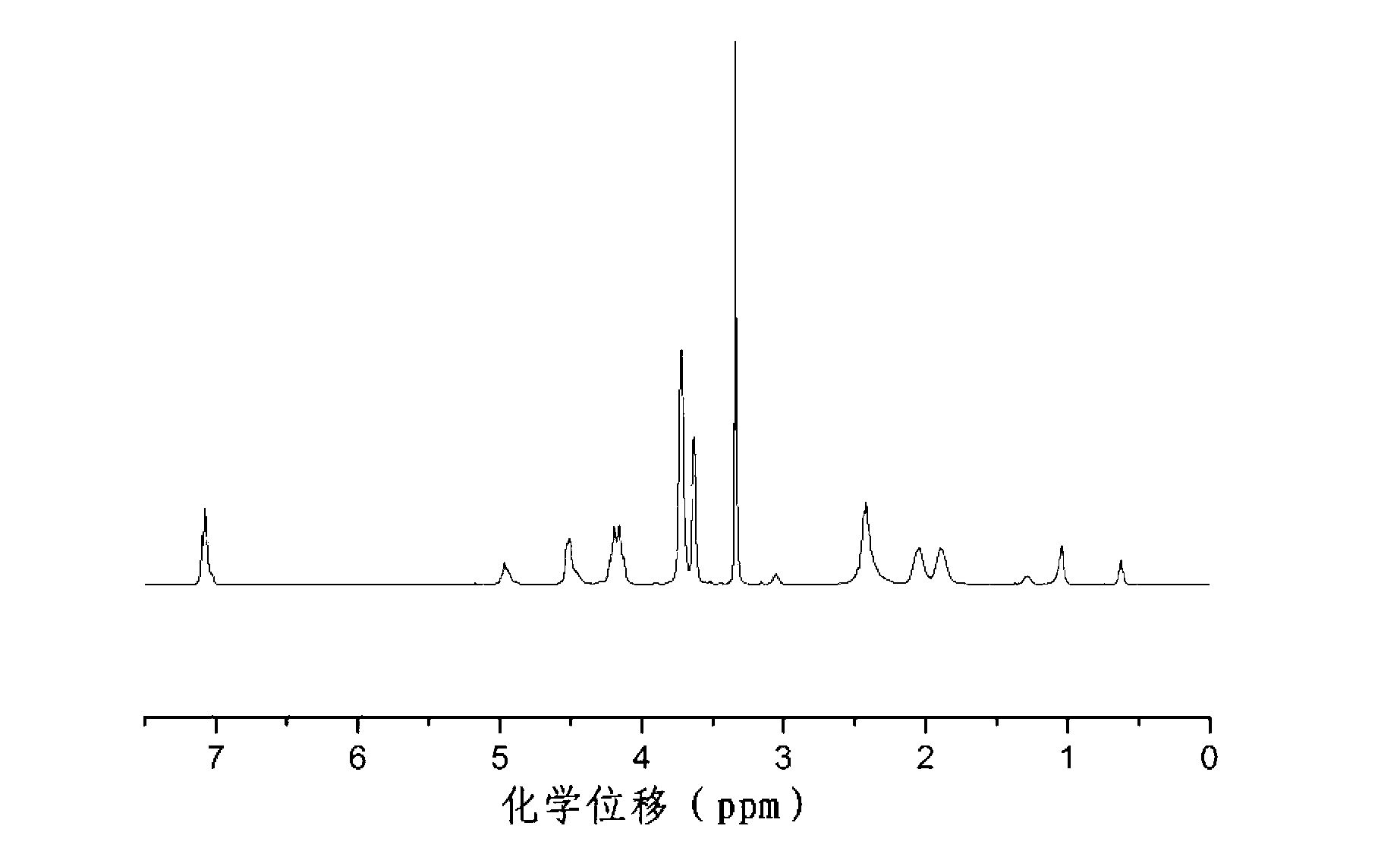
[0062] combine figure 1 Illustrative Example 2
[0063] Preparation of poly(γ-diethylene glycol monomethyl ether-L-glutamate)-poly(γ-benzyl-L-glutamate) diblock copolymers with different number average molecular weights:
[0064] Weigh 0.1376 g (0.0005 mol), 0.2753 g (0.001 mol), 1.3763 g (0.0005 mol), 1.3763 g (0.0005 mol), 1.3763 g (0.0005 mol), 1.3763 g (0.0005 mol), 1.3763 g (0.005 mol ), 2.7525 g (0.01 mol), 5.5050 g (0.02 mol) of γ-diethylene glycol monomethyl ether-L-glutamic acid ester-N- internal carboxylic acid anhydride, put into 9 anhydrous reaction flasks, Add 5 mL of anhydrous dioxane to dissolve the monomer, add 10.119 mg (0.0001 mol) of n-hexylamine under stirring, react the solution at 25°C for 72 hours, and then add 1.3162 g (0.005 mol), 1.3162 g (0.005 mol), 1.3162 g (0.005 mol), 5.2648 g (0.02 mol), 2.6324 g (0.01 mol), 0.2632 g (0.001 mol), 0.1316 g (0.0005 mol), 1.3162 g (0.005 mol), 1.3162 g (0.005 mol) of γ-benzyl-L-glutamate-N-internal car...
Embodiment 3
[0070] Preparation of poly(γ-triethylene glycol monomethyl ether-L-glutamate)-poly(γ-benzyl-L-glutamate) diblock copolymers with different number average molecular weights:
[0071] Weighing quality is 0.1597 g (0.0005 mol), 0.3193 g (0.001 mol), 1.5966 g (0.005 mol), 1.5966 g (0.005 mol), 1.5966 g (0.005 mol), 1.5966 g (0.005 mol), 1.5966 g ( 0.005 mol), 3.1931 g (0.01 mol), 6.3862 g (0.02 mol) of γ-triethylene glycol monomethyl ether-L-glutamic acid ester-N-internal carboxylic acid anhydride, put into 9 anhydrous reaction flasks 5 mL of anhydrous chloroform was added to dissolve the monomer, and 10.119 mg (0.0001 mol) of n-hexylamine were added under stirring, and the solution was stirred at 25°C for 72 hours, and then 1.3162 g (0.005 mol), 1.3162 g (0.005 mol), 1.3162 g (0.005 mol), 5.2648 g (0.02 mol), 2.6324 g (0.01 mol), 0.2632 g (0.001 mol), 0.1316 g (0.0005 mol), 1.3162 g (0.005 mol), 1.3162 g (0.005 mol) of γ-benzyl-L-glutamate-N-internal carboxylic acid anhydride, c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com