Hemostatic powder and preparation method thereof
A technology of hemostatic powder and powder, which is applied in the field of biomedical materials, can solve problems such as abnormal foreign body reaction, reduced hemostatic performance, and inapplicability of hemostatic powder, and achieve strong structural stability, rapid hemostasis, in-situ deposition and adhesion Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
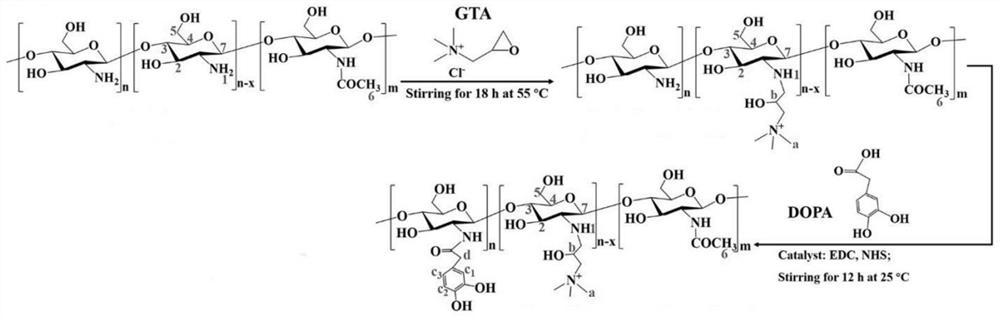
[0052] The preparation of catechol-modified quaternary ammonium salt chitosan (catechol-quaternary ammonium salt chitosan), the preparation principle is as follows figure 1 As shown, a two-step reaction was used to prepare catechol-modified quaternary ammonium salt chitosan.
[0053] first step:
[0054] 5 g of chitosan was weighed and dissolved in 100 mL of HCl solution with a pH of about 4.0 to obtain a chitosan solution;
[0055] 14.1 g of 2,3-epoxypropyltrimethylammonium chloride (Glycidyl trimethylammonium chloride, GTA) was added to the chitosan solution, stirred at 55 °C, and reacted for 18 h to obtain modified chitosan solution;
[0056] The modified chitosan solution was purified in deionized water with a dialysis membrane with a molecular cut-off of 12000 Da for 3 d, and the purified material was taken out and lyophilized to obtain quaternary ammonium salt-modified chitosan.
[0057] Step 2:
[0058] Weigh 3 g of the quaternary ammonium salt-modified chitosan pre...
Embodiment 2
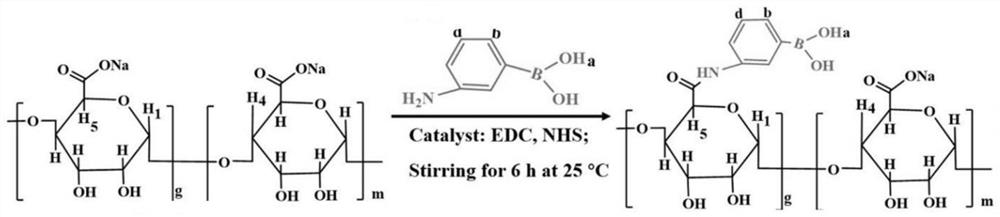
[0063] The preparation of phenylboronic acid-modified sodium alginate (phenylboronic acid-sodium alginate), the preparation principle is as follows figure 2 shown.
[0064] Weigh 3 g of sodium alginate, dissolve it in 300 mL of deionized water, add catalyst and intermediate, stir for 15 min, and mix well to obtain a mixed solution; wherein, the catalyst is 1.305 g of 1-ethyl-3-(3-( Dimethylamino)propyl)carbodiimide hydrochloride (1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide, EDC·HCl), in the middle is 0.675g of N-carboxysuccinimide (N-carbodiimide) hydroxysuccinimide, NHS);
[0065] Then, a solution of 3-aminobenzeneboronic acid (3-aminobenzeneboronic acid) dissolved in dimethyl sulfoxide was added to the prepared mixed solution, wherein the content of 3-aminoboronic acid was 2.151 g, and the acylation reaction was stirred at 25 °C for 12 h;
[0066] The mixed solution after the acylation reaction was dialyzed and purified in deionized water with a dialysis membrane with ...
Embodiment 3
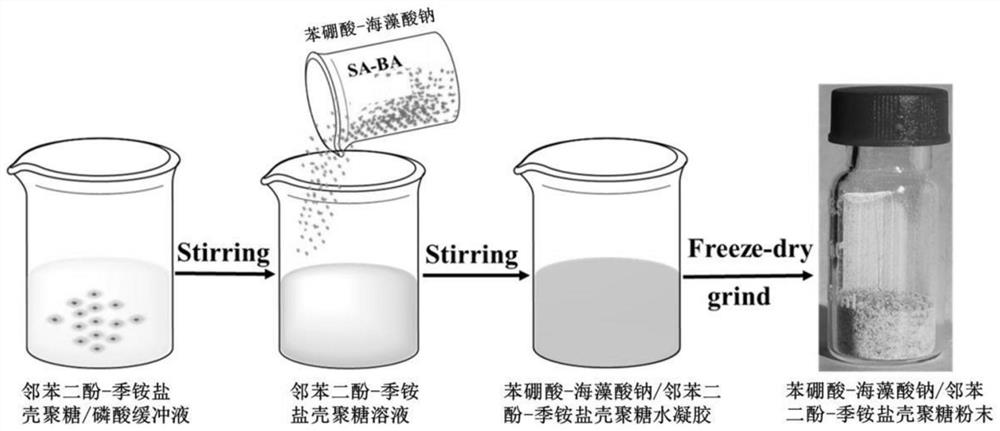
[0069] Preparation of hemostatic powder (1)
[0070] like image 3 and Figure 4 As shown, weigh 1.5 g of catechol-modified quaternary ammonium salt chitosan prepared by the method of Example 1, dissolve it in phosphate buffered saline (PBS) with a pH of about 7.4, and prepare a concentration of 2%. (w / v) catechol-modified quaternary ammonium salt chitosan solution;
[0071] Weigh 4.5 g of phenylboronic acid-modified sodium alginate powder prepared by the method of Example 2, dissolve it in the catechol-modified quaternary ammonium salt chitosan solution, and stir to promote the phenylboronic acid-modified sodium alginate powder. It is fully dissolved and cross-linked with the quaternary ammonium salt chitosan modified by catechol to form a homogeneous hydrogel, and the pH of the hydrogel mixed solution is kept between 7.0 and 7.5;
[0072] Put the hydrogel into a dialysis membrane with a molecular cut-off of 12,000 Da, and dialyze the hydrogel in deionized water for 1 d to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com