Preparation method of drug microspheres taking hydroxyethyl starch 200/0.5 as carrier
A technology of hydroxyethyl starch and microspheres, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical preparations of non-active ingredients, etc., which can solve the problems of prolonging the action time of drugs on lesion tissues, the effect of drug release behavior, and changes in blood drug concentration Large and other problems, to achieve the effect of safe and reliable drug release stability, good drug release stability, and large drug loading capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] A preparation method of drug microspheres using hydroxyethyl starch 200 / 0.5 as a carrier, using lovastatin as a starting material, through H 2 O / O 2 The mixed gas performs plasma chemical modification on the surface of lovastatin, and then coats a layer of hydroxyethyl starch 200 / 0.5 on the surface of lovastatin after surface plasma chemical modification to form hydroxyethyl starch 200 / 0.5 wrapped lovastatin Statin microspheres; the main steps of the preparation method include:
[0019] 1) The plasma chemical modification of the surface of lovastatin is as follows: first, 50 mg of lovastatin is added into the rotatable plasma reaction chamber, and the gas in the plasma reaction chamber is extracted to a pressure of 2Pa; H with a ratio of 1:5 2 O / O 2 Mix the gas to a pressure of 80Pa, repeat the pumping of the gas three times, and finally adjust the pressure in the plasma reaction chamber to 40Pa. Under the conditions of the rotation speed of the plasma reaction chamb...
Embodiment 2~ Embodiment 8
[0022] The difference with Example 1 is that the plasma chemical modification conditions on the surface of lovastatin (shown in Table 1), and hydroxyethyl starch 200 / 0.5 wrapping process on the surface of lovastatin after surface plasma chemical modification Parameters and components (see Table 2).
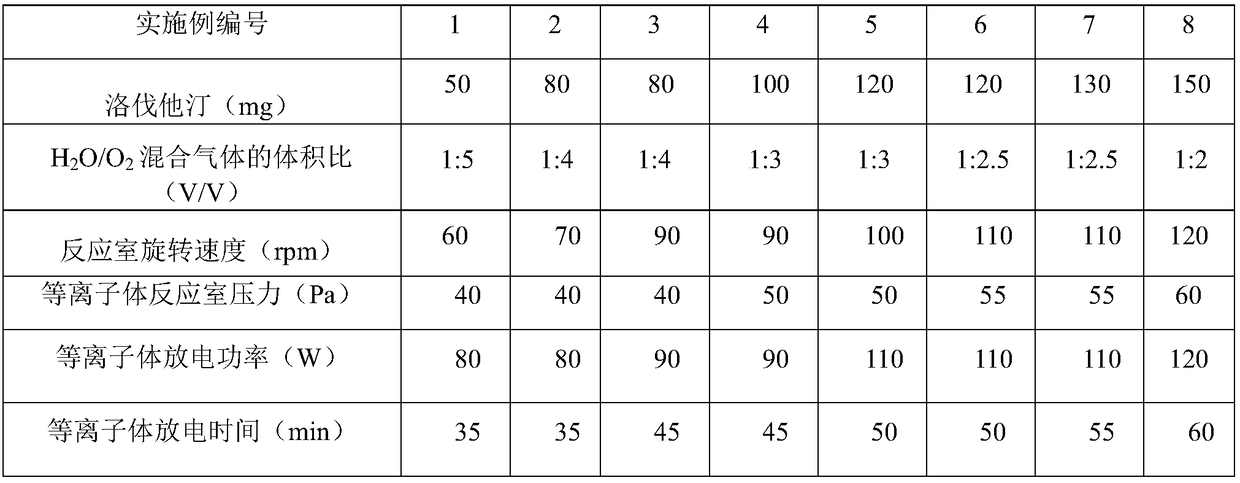
[0023] Plasma chemical modification conditions on the surface of table 1 Examples 1-8 lovastatin
[0024]
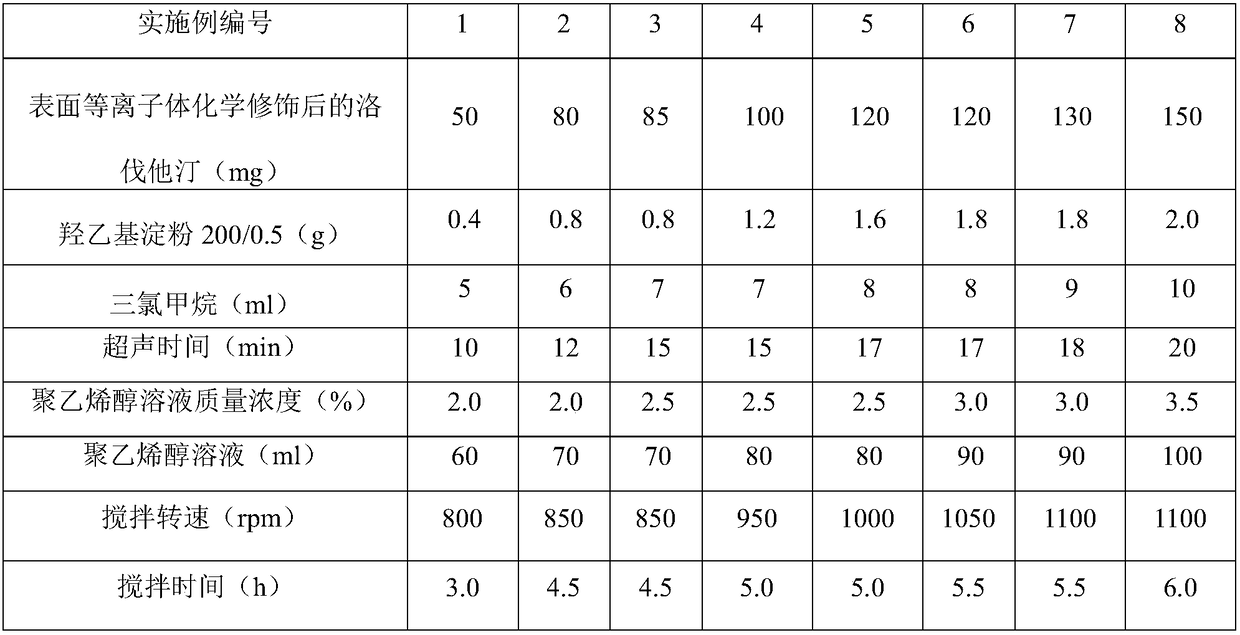
[0025] Table 2 Example 1~8 hydroxyethyl starch 200 / 0.5 on the surface of lovastatin after surface plasmon chemical modification
[0026] Line wrapping process parameters and components
[0027]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com