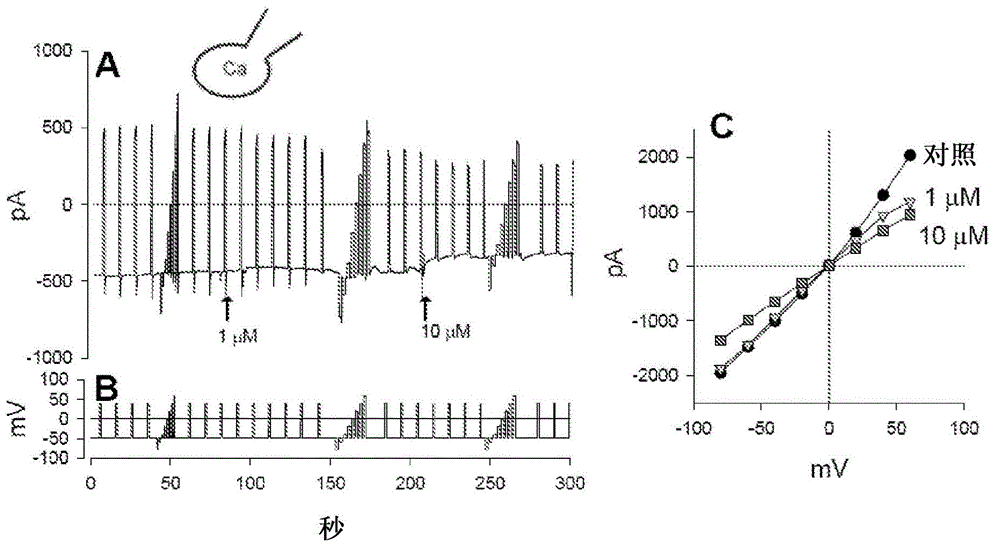
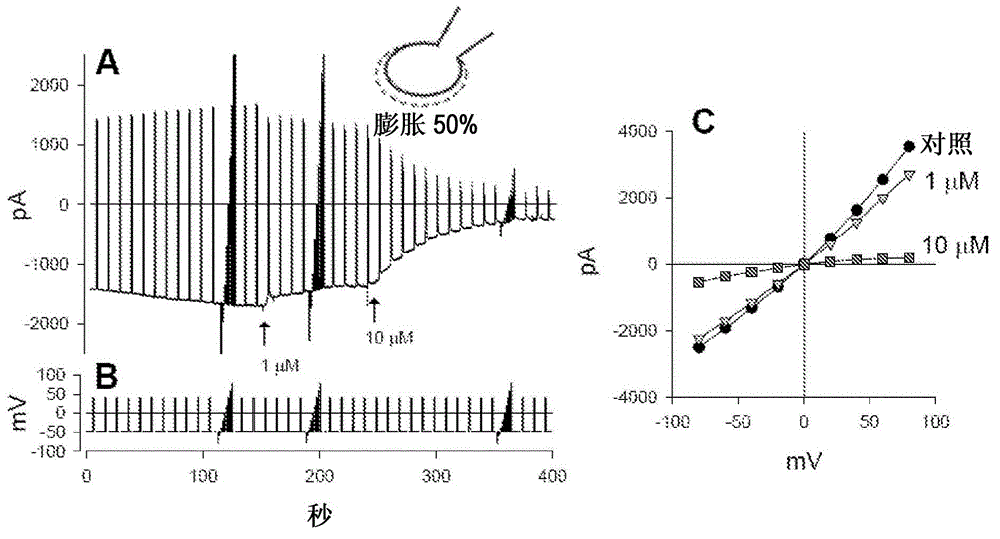
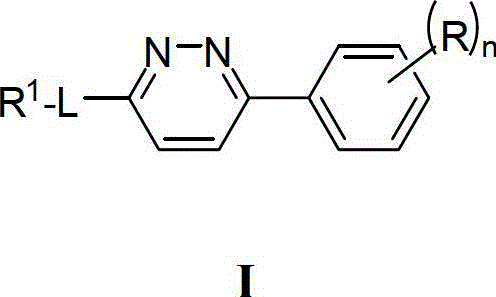
Compounds, compositions and methods comprising pyridazine sulfonamide derivatives
A compound, animal technology, applied in the direction of drug combination, active ingredients of heterocyclic compounds, digestive system, etc., can solve the problem of lack of specific blockers of channels, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0464] N-(3-(6-(4-chlorophenethoxy)pyridazin-3-yl)phenyl)-4-cyanobenzenesulfonamide (compound 3) and N-(3-(6-(benzyl Synthesis of (ethyl)amino)pyridazin-3-yl)phenyl)methanesulfonamide (Compound 7)
[0465]
[0466] Step 1: 3-(4-Chlorophenethoxy)-6-iodopyridazine (compound A)
[0467]To a stirred mixture of 60% sodium hydride mineral oil (0.96 g, 25.0 mmol) in anhydrous THF (30 mL) was added dropwise 4-chlorophenethyl alcohol (3.10 mL) under nitrogen in an ice-water bath at 2 °C , 22.9 mmol). After 30 min a solution of 3-chloro-6-iodopyridazine (5.00 g, 20.8 mmol) in THF (70 mL) was added. The cooling bath was removed and stirring was continued at room temperature for 0.5 h, then at 60 °C for 1.5 h. The mixture was cooled to room temperature and the solvent was removed under vacuum. The residue was partitioned between ethyl acetate (250 mL) and water (150 mL). The combined organic layers were washed with aqueous sodium chloride (150 mL) and dried over a hydrophobic frit...
Embodiment 2
[0477] Preparation of N-(3-(6-(benzyl(ethyl)amino)pyridazin-3-yl)phenyl)-1,1,1-trifluoromethanesulfonamide (compound 10)
[0478]
[0479] Step 1: 6-(3-Aminophenyl)-N-benzyl-N-ethylpyridazin-3-amine (compound D)
[0480] N-benzyl-N-ethyl-6-iodopyridazin-3-amine (100mg, 0.29mmol), 3-aminophenylboronic acid (55.3mg, 0.32mmol), potassium carbonate (0.12g , 0.83 mmol) to a stirred mixture of degassed toluene (2 mL), absolute ethanol (2 mL) and water was added polymer-bound tetrakis(triphenylphosphine)palladium(0) (75 mg, 0.03 mmol, 0.5-0.9 mmol / g load). The mixture was stirred at room temperature for 15 minutes under nitrogen, then heated at 90 °C for 18 h. The mixture was cooled to room temperature, and the solvent was removed under vacuum. The residue was purified by column chromatography (eluent 9:1 to 2:1, hexane:ethyl acetate) to afford 52 mg (49%) of the title compound as a light yellow solid. The crude product was used directly in the next step without further purif...
Embodiment 3
[0488] Preparation of 3-(6-(4-chlorophenethoxy)pyridazin-3-yl)-N-(4-fluorophenylsulfonyl)benzamide (compound 12)
[0489]
[0490] Step 1: 3-(6-(4-chlorophenethoxy)pyridazin-3-yl)benzoic acid (Compound E)
[0491]3-(4-Chlorophenethoxy)-6-iodopyridazine (1.00g, 2.78mmol), 3-carboxyphenylboronic acid (0.51g, 3.06mmol), anhydrous sodium carbonate (1.15 g, 8.34 mmol) To a stirred mixture of degassed toluene (20 mL), absolute ethanol (20 mL) and water (2 mL) was added tetrakis(triphenylphosphine)palladium(0) (0.33 g, 0.28 mmol). The mixture was stirred at room temperature for 15 minutes under nitrogen, then heated at 80 °C for 3 h. The mixture was cooled to room temperature, and the solvent was removed under vacuum. The residue was partitioned between dichloromethane (30 mL) and saturated aqueous sodium bicarbonate (100 mL). The aqueous layer was washed successively with dichloromethane (3 x 30 mL), then acidified to pH 1 (10M HCl, 5 mL). The precipitated solid was dissolved...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com