Method for directly preparing sulfate and ferric oxide by ferrous sulfate
A ferrous sulfate and iron oxide technology, which is applied in the directions of iron oxide, chemical instruments and methods, iron oxide/iron hydroxide, etc., can solve the problems of cumbersome process and high cost of comprehensive utilization of ferrous sulfate, so as to improve economic benefits and save money. cost, effect of reducing environmental emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
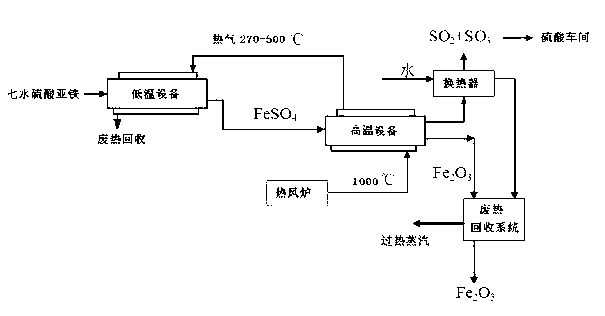
[0051] Put 1 ton of ferrous sulfate heptahydrate into the low-temperature equipment, and dry the ferrous sulfate heptahydrate with hot air with a waste heat temperature of 270-450 °C for 20 to 40 minutes, and then dry the generated anhydrous ferrous sulfate. Transfer to a high temperature equipment, under the condition of isolating air, use 800-900 ℃ hot air to pyrolyze anhydrous ferrous sulfate for 100-160 minutes, and the iron oxide produced by the decomposition is transferred into the heat exchange kettle 5. The cooling water of the boiler is cooled to room temperature to obtain 226Kg of iron fine powder. The produced sulfur dioxide and sulfur trioxide are removed from the sulfuric acid production section to produce 320Kg of 98% sulfuric acid. The superheated steam from the heat exchange kettle is removed from the titanium dioxide hydrolysis section. Rotary kiln is used for low temperature equipment, and drum heating equipment is used for high temperature equipment.
Embodiment 2
[0053] Put 1 ton of ferrous sulfate heptahydrate into the low-temperature equipment, and dry the ferrous sulfate heptahydrate for 40 to 60 minutes with hot air with a waste heat temperature of 400-500 °C generated by the jacket of the high-temperature equipment, and then dry the generated anhydrous ferrous sulfate. Transfer to high temperature equipment, under the condition of isolating air, use hot air at 850-950 ℃ to pyrolyze anhydrous ferrous sulfate for 30-60 minutes, the iron oxide produced by decomposition is transferred into the heat exchange kettle, The cooling water is cooled to room temperature to obtain 226Kg of iron fine powder, the produced sulfur dioxide and sulfur trioxide are removed from the sulfuric acid production section to produce 320Kg of 98% sulfuric acid, and the superheated steam from the heat exchange kettle is removed from the titanium dioxide hydrolysis section. The low temperature equipment adopts the drum type heating equipment, and the high temper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com