Method for processing and producing potassium chloride by using magnesium sulfate subtype or sulfate chloride transitional-type potassium-containing salt lake brine
A kind of magnesium sulfate isoform and chloride technology, applied in the direction of alkali metal chloride, etc., can solve the problems of poor potassium chloride product quality, low potassium yield and the like, and achieve the effects of good product quality and high potassium salt yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
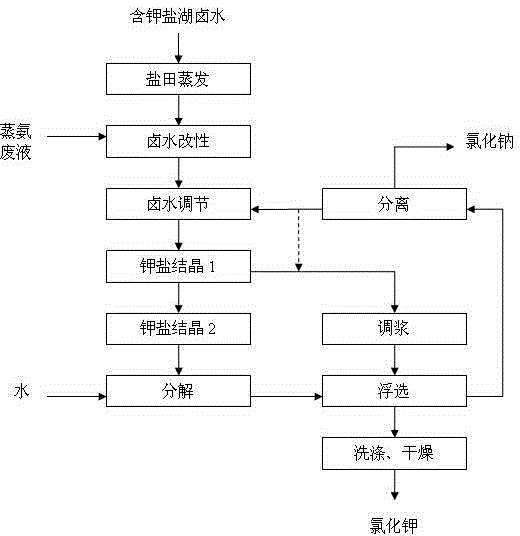
[0015] Embodiment 1, with reference to figure 1 , a method for processing and producing potassium chloride with magnesium sulfate subtype or sulfate chloride transition type potassium-containing salt lake brine, the specific steps are as follows:
[0016] (1) Raw material magnesium sulfate subtype potassium-containing salt lake brine or sulfate chloride transition type potassium-containing salt lake brine contains Na + 、K + , Mg 2+ , Cl - , SO 4 2- ions, and where SO 4 2- with K + The weight ratio is 0.5:1; the modifying agent is introduced in the halite stage of the brine evaporation process, that is, the modifying agent is introduced when it precipitates 0% sodium chloride, so that the SO in the brine 4 2- Pre-precipitation of ions; the amount of modifier added to control the modified brine SO 4 2- :K + The weight ratio is 0.1:1, after the crystalline gypsum and halite are precipitated, the modified brine is separated, and the modified brine continues to be sun-d...
Embodiment 2
[0018] Embodiment 2, with reference to figure 1 , a method for processing and producing potassium chloride with magnesium sulfate subtype or sulfate chloride transition type potassium-containing salt lake brine, the specific steps are as follows:
[0019] (1) Raw material magnesium sulfate subtype potassium-containing salt lake brine or sulfate chloride transition type potassium-containing salt lake brine contains Na + 、K + , Mg 2+ , Cl - , SO 4 2- ions, and where SO 4 2- with K + The weight ratio is 3.5:1; the modifier is introduced in the halite stage of the brine evaporation process, that is, the modifier is introduced when it precipitates 85% of sodium chloride, so that the SO in the brine 4 2- Pre-precipitation of ions; the amount of modifier added to control the modified brine SO 4 2- :K + The weight ratio is 2.0:1, after the crystalline gypsum and halite are precipitated, the modified brine is separated, and the modified brine continues to be sun-dried and e...
Embodiment 3
[0021] Embodiment 3, with reference to figure 1 , a method for processing and producing potassium chloride with magnesium sulfate subtype or sulfate chloride transition type potassium-containing salt lake brine, the specific steps are as follows:
[0022] (1) Raw material magnesium sulfate subtype potassium-containing salt lake brine or sulfate chloride transition type potassium-containing salt lake brine contains Na + 、K + , Mg 2+ , Cl - , SO 4 2- ions, and where SO 4 2- with K + The weight ratio of the brine is 2.0:1; the modifier is introduced in the halite stage of the brine evaporation process, that is, the modifier is introduced when 50% of the sodium chloride is precipitated, so that the SO in the brine 4 2- Pre-precipitation of ions; the amount of modifier added to control the modified brine SO 4 2- :K + The weight ratio is 1.5:1, after the crystalline gypsum and halite are precipitated, the modified brine is separated, and the modified brine continues to b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com