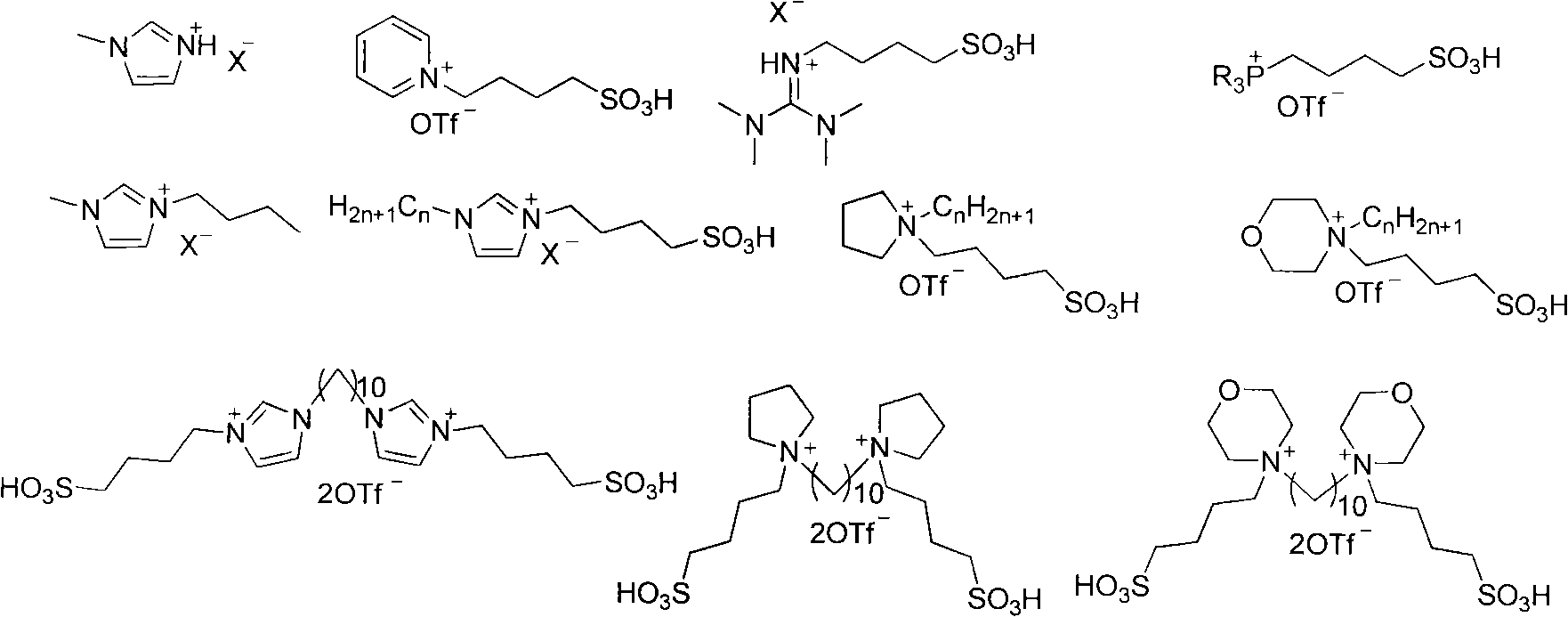
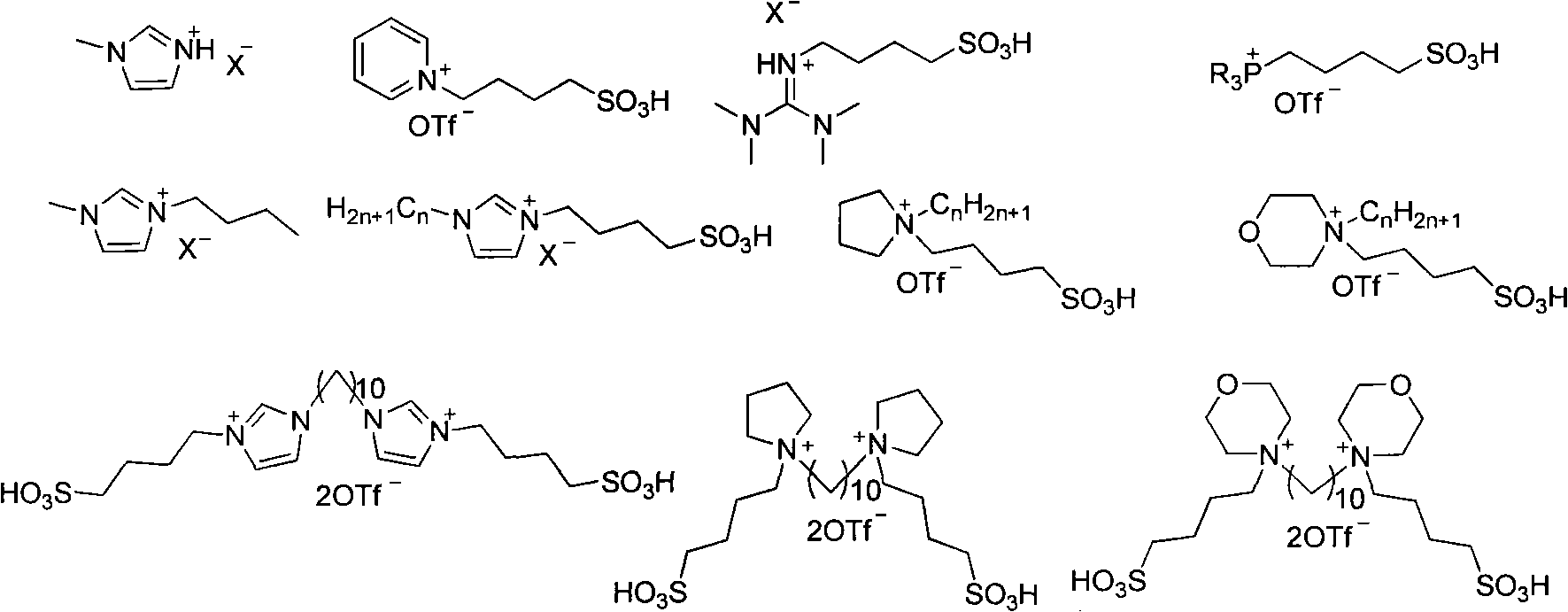
Method for synthesizing amine compound catalyzed by functionalized ionic liquid
A technology of ionic liquids and compounds, which is applied in the field of synthesis of amine compounds, can solve the problems of high price and unfriendly environment, and achieve the effects of simple operation, simple post-treatment and good catalyst stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] At 80°C, p-toluenesulfonamide (0.75mmol, 128.3mg) and diphenylmethanol (0.5mmol, 92.0mg) and [BsTdmim]OTf (20mol%, 55.0mg), 1,4-dioxane 2.0mL was placed in a dry reaction bottle, and stirred by magnetic force for 3h. After the reaction was completed, column chromatography separated (using a silica gel column, eluent: petroleum ether / ethyl acetate=5 / 1) to obtain 154.9 mg of white solid N-benzhydryl-4-methylbenzenesulfonamide, producing The rate is 91.9%. 1 H NMR (400MHz, CDCl 3 )δ=2.37(s, 3H), 5.15(d, J=6.8Hz, 1H), 5.56(d, J=6.8Hz, 1H), 7.09-7.21(m, 12H), 7.56(d, J=8.0 Hz, 2H). 13 C NMR (100MHz, CDCl 3)δ=21.5, 61.4, 127.2, 127.4, 127.6, 128.5, 129.4, 137.3, 140.5, 143.2. HRMS-ESI: Calcd. For C 20 h 19 NNaO 2 S[M+Na] + :360.1029.Found: 360.1020.
Embodiment 2
[0036] With example 1, used catalyst is [TG][OTf], the productive rate of N-benzhydryl-4-methylbenzenesulfonamide is 79.3%.
Embodiment 3
[0038] With example 1, used catalyst is [LPS][OTf], the productive rate of N-benzhydryl-4-methylbenzenesulfonamide is 61.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com