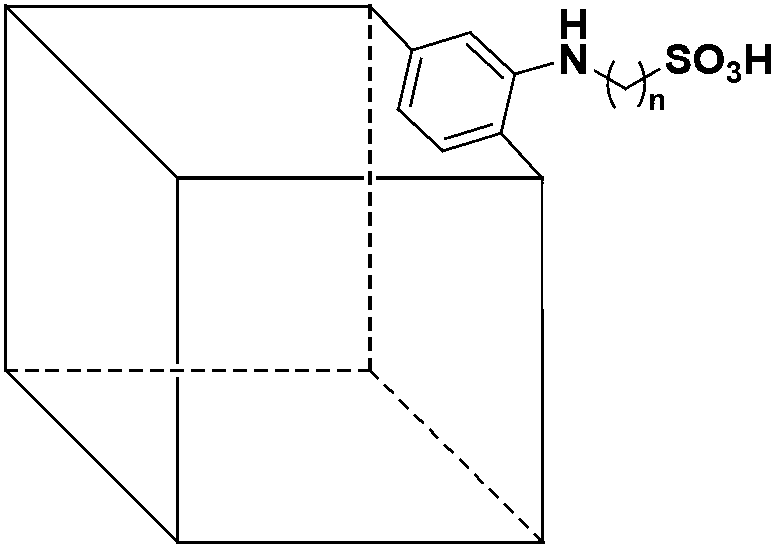
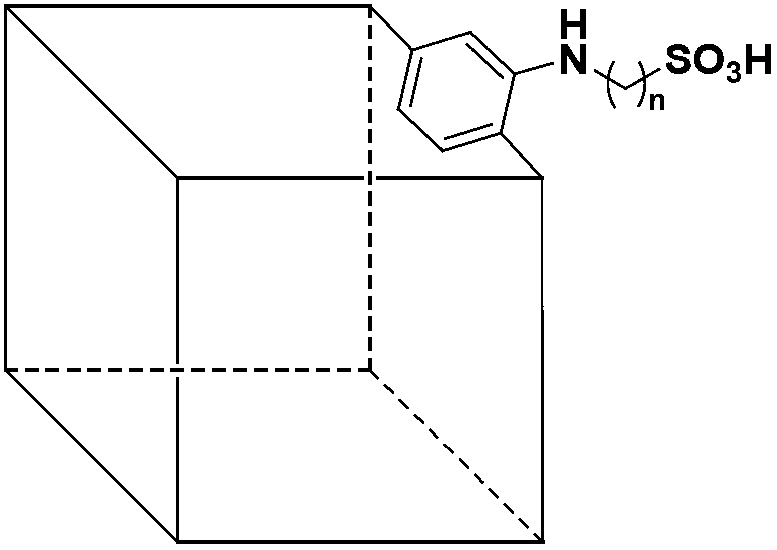
Method for catalytic esterification of -SO3H-containing metal-organic framework compound
A metal-organic framework, -SO3H technology, applied in the preparation of organic compounds, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., can solve problems such as difficult product separation, harsh reaction conditions, and large losses , to achieve the effect of strong controllability, simple separation and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1: 10 mmol of n-butanol, 10 mmol of glacial acetic acid and 16 mg of IRMOF-3a were added to a 25 ml one-necked flask with a stirring bar and a reflux condenser. The reaction was vigorously stirred at 110°C for 45 minutes, cooled to room temperature and filtered. The filtrate was detected by gas chromatography with a conversion rate of 98%, a selectivity of 100%, and a yield of 98%. The filter residue was vacuum-dried at 120°C for 8 hours to remove water and then recycled.
Embodiment 2
[0016] Example 2: 10 mmol of n-octanol, 15 mmol of glacial acetic acid and 16 mg of IRMOF-3a were respectively added to a 25 ml one-necked flask equipped with a stirring bar and a reflux condenser. The reaction was vigorously stirred at 110°C for 60 minutes, cooled to room temperature and filtered. The filtrate was detected by gas chromatography with a conversion rate of 94%, a selectivity of 100%, and a yield of 94%. The filter residue was vacuum-dried at 120°C for 8 hours to remove water and then recycled.
Embodiment 3
[0017] Example 3: 20mol ethanol, 10mmol oxalic acid and 36mg IRMOF-3b were respectively added into a 25ml single-necked bottle with a stirring bar and a reflux condenser. The reaction was vigorously stirred at 110°C for 60 minutes, cooled to room temperature and filtered. The filtrate was detected by gas chromatography with a conversion rate of 92%, a selectivity of 100%, and a yield of 92%. The filter residue was vacuum-dried at 120°C for 8 hours to remove water and then recycled.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com