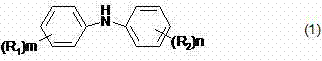
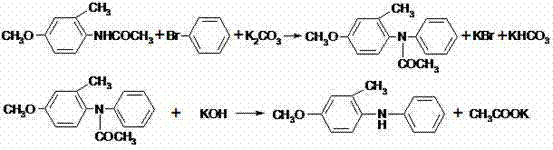
Preparation method of diphenylamine or ring-substituted derivative thereof
A ring-substituted, diphenylamine technology is applied in the field of preparing diphenylamine or its ring-substituted derivatives, and can solve the problems of not achieving energy saving, consumption reduction, pollution reduction, efficiency enhancement and clean production process, shortening reaction time, etc. , to achieve the effect of improving equipment utilization, shortening the reaction cycle and simplifying the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Add 201.5g (1.0mol) of 4-bromo-3-methylanisole, 141.8g (1.05mol) of acetanilide, and 3.8g (0.038mol) of cuprous chloride into the reactor, and start heating while stirring , when the temperature of the material in the reactor reaches 200°C, start to add 234.0g (1.3mol) of 30wt% sodium methoxide methanol solution dropwise for 8 hours, and keep the temperature of the reaction material at 200°C during the dropping process. After the dropwise addition, the insulation reaction was continued for 0.5 hour, and the reaction ended. Obtained methanol and methyl acetate mixture fraction 207.3g, methyl acetate content 26.2% (gas chromatography analysis).
[0067] Add 200g of water and 500ml of toluene to the reaction mixture containing 4-methoxy-2-methyldiphenylamine to dissolve the soluble substances in the system, filter to remove the insoluble substances, separate the toluene layer, and then extract the water layer with 300ml of toluene 2 times, the toluene layer was combined w...
Embodiment 2
[0069] As described in Example 1, the difference is that 10% of the total amount of 4-bromo-3-methylanisole, that is, 20.2g (0.1mol), is added to the reactor simultaneously with acetanilide, and the remaining 90%, that is, 181.3 g (0.9mol) and 234.0g (1.3mol) of 30wt% methanol solution of sodium methoxide were added dropwise at the same time, and the rate of addition was controlled. The time for adding 4-bromo-3-methylanisole was 6 hours, and the sodium methoxide solution The dropwise addition time was 6.5 hours.
[0070] 188.0 g of the target product 4-methoxy-2-methyldiphenylamine was obtained, with a purity of 99.3% (gas chromatography analysis), and a reaction yield of 87.6%. Methanol and methyl acetate mixture distillate 195.5g, methyl acetate content 28.2% (gas chromatography analysis).
Embodiment 3
[0072] As described in Example 1, the difference is that the methanol solution of 30 wt% sodium methoxide is 198.0 g (1.1 mol). 175.4 g of the target product 4-methoxy-2-methyldiphenylamine was obtained, with a purity of 99.2% (gas chromatography analysis), and a reaction yield of 81.7%. Methanol and methyl acetate mixture distillate 184.7g, methyl acetate content 24.2% (gas chromatography analysis).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com